Why Do Ions Travel Back And Forth In Orbitrap
Muz Play
Mar 17, 2025 · 5 min read
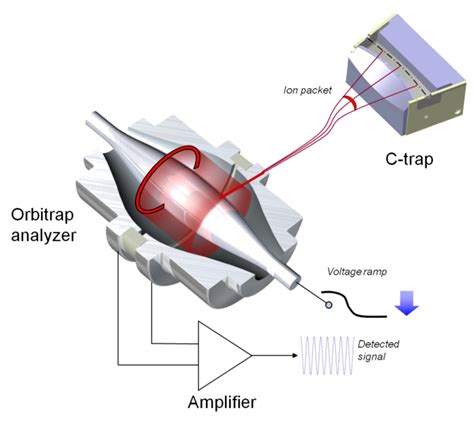
Table of Contents
Why Do Ions Travel Back and Forth in an Orbitrap? Understanding Ion Motion in Orbitrap Mass Spectrometry
Orbitrap mass spectrometry has revolutionized the field of analytical chemistry, offering unparalleled mass accuracy and resolution. At the heart of this technology lies the orbitrap itself – a unique device where ions are trapped and their oscillatory motion is precisely measured to determine their mass-to-charge ratio (m/z). But why do these ions execute this characteristic back-and-forth motion? Understanding this fundamental principle is crucial to appreciating the power and precision of orbitrap mass spectrometry.
The Orbitrap: A High-Precision Ion Trap
The orbitrap is essentially a highly precise ion trap. It consists of a central, spindle-shaped electrode surrounded by a barrel-shaped electrode. A high DC voltage is applied between these two electrodes, creating a strong electrostatic field. This field is what governs the ions' movement. Ions injected into this field don't simply sit still; they begin to oscillate around the central electrode in a complex, yet predictable, manner.
The Role of Electrostatic Forces
The key to understanding ion motion within the orbitrap lies in understanding the interplay of electrostatic forces. The DC voltage creates a radial electric field that pulls ions towards the central electrode. However, the ions also possess initial kinetic energy, gained during their introduction into the orbitrap. This kinetic energy translates into a tangential velocity, causing the ions to move around the central electrode in a circular or elliptical path.
The combination of the radial pull towards the central electrode and the tangential motion results in a complex oscillatory trajectory. The ions don't simply move in a perfect circle; they also move back and forth along the central axis, creating the characteristic "back-and-forth" motion we observe. This movement is not chaotic; it's governed by precise physical laws, and this predictability is what allows for accurate mass determination.
Decomposing the Ion Motion: Axial and Radial Oscillations
To fully grasp the ion's trajectory, it's helpful to break down the motion into two components: axial oscillation (movement along the central axis) and radial oscillation (movement perpendicular to the central axis).
Axial Oscillation: The "Back-and-Forth" Motion
The axial oscillation is the back-and-forth movement that's so characteristic of the orbitrap. This motion is caused by the variation in the radial electric field along the central axis. The field is stronger closer to the central electrode and weaker further away. As ions move towards the central electrode, they experience a stronger pull, which slows them down and eventually reverses their direction. They then oscillate back and forth along the central axis, continually experiencing this alternating pull.
The frequency of this axial oscillation is directly related to the ion's m/z ratio. Heavier ions (higher m/z) oscillate more slowly than lighter ions (lower m/z). This crucial relationship forms the foundation of mass determination in orbitrap mass spectrometry.
Radial Oscillation: The Circular or Elliptical Component
Simultaneously with the axial oscillation, ions also execute a radial oscillation. This is the movement around the central electrode. The strength of the radial electric field combined with the ions' initial tangential velocity determines the shape of this radial oscillation – it can be a circle, an ellipse, or even more complex patterns. While important for the overall trajectory, the radial oscillation's frequency is not directly used for mass determination.
The Influence of Ion Cloud Dynamics
The description above simplifies the scenario by focusing on a single ion. In reality, an orbitrap contains a cloud of ions, each with slightly different m/z ratios and initial velocities. These ions interact with each other through Coulombic repulsion, which introduces a level of complexity to the system. The ions' mutual repulsion can slightly perturb their individual trajectories.
However, the overall dynamics of the ion cloud remain remarkably stable. The high precision of the electrostatic field and the inherent stability of the oscillation frequencies minimize the effects of these inter-ionic interactions, ensuring that the mass measurement remains accurate. Sophisticated mathematical models account for these effects, further enhancing the accuracy of mass determination.
Image Current Detection: Measuring the Ion's Oscillation
The detection process in an orbitrap is ingenious. The ions' oscillatory motion induces a tiny image current in the external detection electrodes. This image current is a direct reflection of the ions' collective motion, encoding crucial information about their m/z ratios. The frequency of this image current directly correlates to the frequency of the ions' axial oscillation, and hence their m/z ratio.
High-resolution Fourier transform algorithms are employed to analyze the image current signal. These algorithms efficiently decompose the complex waveform into its constituent frequencies, revealing the presence of individual ions and their respective m/z ratios with exceptional accuracy. The exceptional resolution stems from the ability to accurately measure minute differences in oscillation frequencies.
Advantages of the Orbitrap's Back-and-Forth Motion
The unique back-and-forth motion of ions within the orbitrap offers several significant advantages in mass spectrometry:
-
High Mass Accuracy: The precise relationship between axial oscillation frequency and m/z enables highly accurate mass measurements.
-
High Resolution: The ability to distinguish between ions with very similar m/z ratios leads to high resolution.
-
High Sensitivity: The efficient trapping of ions and the sensitive detection method contribute to high sensitivity.
-
Versatility: Orbitraps are compatible with various ionization techniques and applications.
Conclusion: A Symphony of Electrostatic Forces
The back-and-forth motion of ions in an orbitrap isn't merely a curious phenomenon; it's the cornerstone of this powerful mass spectrometry technique. This motion, a consequence of the interplay between electrostatic forces and the ions' kinetic energy, is precisely measured to determine their mass-to-charge ratio with remarkable accuracy. The high mass accuracy, high resolution, and high sensitivity of orbitrap mass spectrometry all stem from this elegant interplay of forces and the sophisticated algorithms used to interpret the resulting image current. This intricate dance of ions provides invaluable insights into complex biological systems, environmental samples, and many other areas of scientific inquiry, securing the orbitrap's position as a leading technology in mass spectrometry. Further research into optimizing ion motion and improving signal processing continues to push the boundaries of what's achievable with this revolutionary technology. The understanding of these fundamental principles allows for continued innovation and advancement in the field of mass spectrometry.
Latest Posts
Latest Posts
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Why Do Ions Travel Back And Forth In Orbitrap . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
