A Neutron Has Approximately The Same Mass As
Muz Play
Mar 25, 2025 · 6 min read
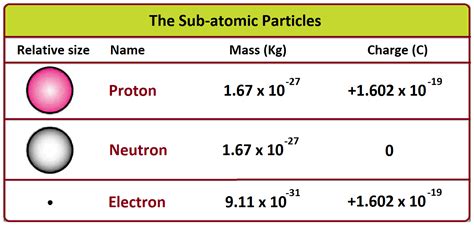
Table of Contents
A Neutron Has Approximately the Same Mass As: Delving into the World of Subatomic Particles
The question, "A neutron has approximately the same mass as..." might seem simple at first glance. However, understanding the answer requires delving into the fascinating world of subatomic particles, their properties, and the intricate relationships within the atomic nucleus. This article will explore the mass of a neutron, comparing it to other fundamental particles and examining the implications of this mass equivalence in various scientific contexts.
Understanding the Neutron's Mass
A neutron is a subatomic particle, a fundamental constituent of atomic nuclei. It's characterized by its neutral charge (hence the name "neutron") and a mass slightly greater than that of a proton. The mass of a neutron is approximately 1.674927471 × 10^-27 kilograms, or about 939.565 MeV/c² (Mega-electronvolts per speed of light squared) using Einstein's famous mass-energy equivalence equation, E=mc². This seemingly small mass has profound implications for the stability and behavior of atomic nuclei.
Comparing the Neutron's Mass to Other Particles
The most frequently encountered comparison is with the proton. A proton, also a constituent of the atomic nucleus, carries a positive charge and has a mass remarkably close to that of the neutron. The proton's mass is approximately 1.6726219 × 10^-27 kilograms, or 938.272 MeV/c². The difference in mass between a proton and a neutron is relatively small, only about 0.14%, yet this seemingly insignificant difference has crucial consequences in nuclear processes like beta decay.
Another relevant comparison is with the electron. Electrons, significantly lighter than both protons and neutrons, are negatively charged particles that orbit the atomic nucleus. An electron's mass is approximately 9.10938356 × 10^-31 kilograms, or 0.511 MeV/c². This stark contrast in mass highlights the dominance of protons and neutrons in determining an atom's mass. The mass of electrons contributes negligibly to the overall mass of an atom.
The mass difference between a proton and a neutron is not merely an interesting fact; it's central to understanding nuclear stability and radioactive decay. The slightly higher mass of the neutron allows it to decay into a proton, an electron (beta particle), and an electron antineutrino through a process governed by the weak nuclear force. This decay is responsible for certain types of radioactivity.
The Significance of Neutron Mass in Nuclear Physics
The neutron's mass plays a pivotal role in numerous nuclear phenomena. Its mass, slightly higher than the proton, affects:
1. Nuclear Stability:
The ratio of neutrons to protons in an atomic nucleus is crucial for its stability. Too many or too few neutrons can lead to an unstable nucleus prone to radioactive decay. This neutron-proton ratio is influenced directly by the mass difference between these particles. Heavier elements generally require a higher neutron-to-proton ratio for stability.
2. Nuclear Reactions:
Neutron mass significantly influences the energy changes involved in various nuclear reactions, such as fission and fusion. The mass difference between reactants and products in a nuclear reaction is converted into energy according to Einstein's famous equation, E=mc². In nuclear fission, for example, the splitting of a heavy nucleus into lighter nuclei releases a significant amount of energy because the total mass of the products is less than the mass of the original nucleus. This mass difference is primarily due to changes in the arrangement of protons and neutrons.
3. Neutron Stars:
Neutron stars are incredibly dense celestial objects formed from the remnants of massive stars after supernova explosions. These stars are primarily composed of tightly packed neutrons, highlighting the immense gravitational forces needed to overcome the inherent repulsion between these particles. The neutron's mass is a critical factor in determining the properties and behavior of neutron stars, including their size, density, and gravitational field.
Mass-Energy Equivalence and the Neutron
Einstein's famous equation, E=mc², demonstrates the equivalence of mass and energy. This equivalence is particularly relevant for neutrons because their mass can be converted into energy, and vice-versa. In nuclear reactions, a small change in mass corresponds to a large release or absorption of energy. The neutron's mass, therefore, isn't just a static property; it's a measure of its potential energy content.
Implications of Mass-Energy Equivalence in Nuclear Reactions
The slight difference in mass between the neutron and the proton directly translates into energy released during beta decay. The excess mass of the neutron is converted into the kinetic energy of the resulting proton, electron, and antineutrino. Similarly, in nuclear fission, the decrease in total mass from the original nucleus to the fission products corresponds to a vast release of energy, responsible for the power of nuclear weapons and nuclear reactors.
The Neutron's Role in Other Scientific Fields
Beyond nuclear physics, the neutron's mass and properties play a role in other scientific fields:
1. Neutron Activation Analysis:
Neutron activation analysis (NAA) is a highly sensitive analytical technique used to determine the elemental composition of materials. This method utilizes the interaction of neutrons with atomic nuclei, causing them to become radioactive isotopes. The characteristics of the emitted radiation are then used to identify the elements present. The neutron's mass and its ability to induce radioactivity are central to this analytical technique.
2. Neutron Scattering:
Neutron scattering is a powerful technique used to study the structure and dynamics of materials at the atomic and molecular level. Neutrons, owing to their neutral charge and suitable mass, can penetrate deeply into materials and interact with atomic nuclei, providing information about the arrangement and movement of atoms within the sample.
3. Medical Applications:
Neutron radiation, while potentially harmful, also has medical applications in cancer radiotherapy. High-energy neutrons can damage cancerous cells, leading to their destruction. The mass and energy of the neutrons are carefully controlled to target the cancerous tissue while minimizing damage to surrounding healthy tissue.
Conclusion: The Neutron's Mass – A Fundamental Parameter
The answer to "A neutron has approximately the same mass as..." is not a simple "proton". While the mass is remarkably similar to the proton's, the small difference is crucial for understanding many fundamental processes in the universe. The neutron's mass is a fundamental parameter influencing nuclear stability, nuclear reactions, the behavior of neutron stars, and various scientific applications. Its mass, in conjunction with Einstein's mass-energy equivalence, underpins the immense energy released in nuclear processes. The subtle difference between the neutron and proton masses, seemingly insignificant at first glance, is a key to understanding a vast array of physical phenomena, from radioactive decay to the formation of neutron stars. This understanding continues to drive advancements in various scientific and technological fields.
Latest Posts
Latest Posts
-
Which Muscle Is Not Part Of The Rotator Cuff
Mar 28, 2025
-
How Many Neutrons Does Sulfur Have
Mar 28, 2025
-
Which Elements Usually Lose Their Valence Electrons When They Bond
Mar 28, 2025
-
A Particle With A Negative Charge
Mar 28, 2025
-
What Is The Smallest Part Of An Atom
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about A Neutron Has Approximately The Same Mass As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
