How Many Neutrons Does Sulfur Have
Muz Play
Mar 28, 2025 · 5 min read
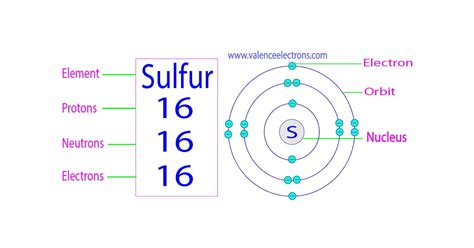
Table of Contents
- How Many Neutrons Does Sulfur Have
- Table of Contents
- How Many Neutrons Does Sulfur Have? A Deep Dive into Isotopes and Atomic Structure
- Understanding Atomic Structure: Protons, Neutrons, and Electrons
- Isotopes: The Key to Variable Neutron Counts
- Atomic Mass and Isotopic Abundance
- Beyond Stable Isotopes: Radioactive Sulfur Isotopes
- The Significance of Isotopic Variations in Sulfur
- Applications of Sulfur and its Isotopes
- Conclusion: A Deeper Appreciation for Sulfur's Complexity
- Latest Posts
- Latest Posts
- Related Post
How Many Neutrons Does Sulfur Have? A Deep Dive into Isotopes and Atomic Structure
Sulfur, a vibrant yellow nonmetal crucial to life and industry, presents a fascinating case study in atomic structure. A simple question, "How many neutrons does sulfur have?", unveils a deeper understanding of isotopes, atomic mass, and the complexities of elemental composition. This exploration will delve into the intricacies of sulfur's neutron count, providing a comprehensive overview accessible to both students and enthusiasts.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into sulfur's neutron number, let's establish a fundamental understanding of atomic structure. Every atom comprises three subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines an element's atomic number and its identity. For sulfur (S), the atomic number is 16, meaning every sulfur atom possesses 16 protons.
-
Neutrons: Neutrally charged particles also located within the nucleus. Unlike protons, the number of neutrons in an element can vary, leading to the existence of isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells. In a neutral atom, the number of electrons equals the number of protons.
Isotopes: The Key to Variable Neutron Counts
The crux of the question, "How many neutrons does sulfur have?", lies in the concept of isotopes. Isotopes are atoms of the same element (same number of protons) but with varying numbers of neutrons. This variation in neutron count affects the atom's mass but not its chemical properties.
Sulfur has several naturally occurring isotopes, each with a different number of neutrons:
-
Sulfur-32 (³²S): This is the most abundant isotope of sulfur, comprising approximately 95% of naturally occurring sulfur. It has 16 protons and 16 neutrons (32 - 16 = 16).
-
Sulfur-33 (³³S): A less abundant isotope, it has 16 protons and 17 neutrons (33 - 16 = 17).
-
Sulfur-34 (³⁴S): Another stable isotope, it contains 16 protons and 18 neutrons (34 - 16 = 18).
-
Sulfur-36 (³⁶S): The rarest stable isotope of sulfur, it possesses 16 protons and 20 neutrons (36 - 16 = 20).
Therefore, there's no single answer to "How many neutrons does sulfur have?" The number of neutrons varies depending on the specific isotope.
Atomic Mass and Isotopic Abundance
The atomic mass of an element listed on the periodic table is a weighted average of the masses of all its naturally occurring isotopes, taking into account their relative abundances. Sulfur's atomic mass is approximately 32.06 atomic mass units (amu). This average reflects the predominance of Sulfur-32.
The weighted average calculation considers the mass and abundance of each isotope:
(Abundance of ³²S * Mass of ³²S) + (Abundance of ³³S * Mass of ³³S) + (Abundance of ³⁴S * Mass of ³⁴S) + (Abundance of ³⁶S * Mass of ³⁶S) = Average Atomic Mass
While the average atomic mass provides a useful representation, it's crucial to remember that individual sulfur atoms have a specific number of neutrons, depending on their isotopic identity.
Beyond Stable Isotopes: Radioactive Sulfur Isotopes
In addition to the stable isotopes mentioned above, sulfur also possesses several radioactive isotopes. These isotopes are unstable and undergo radioactive decay, transforming into other elements over time. These radioactive isotopes have even more variations in neutron count. Examples include Sulfur-35 (³⁵S), used in various scientific applications, which has 16 protons and 19 neutrons.
The study of radioactive isotopes provides valuable insights into various fields, including:
-
Medicine: Radioactive isotopes are used in diagnostic imaging and radiotherapy.
-
Environmental science: Tracing the movement of sulfur compounds in ecosystems.
-
Archaeology: Dating ancient artifacts and materials.
The Significance of Isotopic Variations in Sulfur
The variations in sulfur's isotopic composition have significant implications across multiple scientific disciplines. These variations can be used to:
-
Trace pollution sources: Identifying the origin of sulfur dioxide emissions.
-
Understand geological processes: Studying the formation of sedimentary rocks and mineral deposits.
-
Study biochemical pathways: Tracking sulfur metabolism in living organisms.
Analyzing the isotopic ratios of sulfur in different samples allows researchers to unravel complex processes and gain a deeper understanding of natural and human-influenced systems.
Applications of Sulfur and its Isotopes
Sulfur's diverse properties and the availability of different isotopes make it indispensable in a vast array of applications. These include:
-
Industry: The production of sulfuric acid, a cornerstone chemical used in various industrial processes.
-
Agriculture: Sulfur is an essential nutrient for plant growth, contributing to protein synthesis.
-
Medicine: Used in the production of certain drugs and as a component of some medical treatments.
-
Cosmetics: Used in some skincare and hair care products.
The specific isotopic composition of sulfur might be relevant in some of these applications, influencing the properties of materials and their behaviour in different processes.
Conclusion: A Deeper Appreciation for Sulfur's Complexity
The seemingly simple question, "How many neutrons does sulfur have?", unveils a rich tapestry of atomic structure, isotopic variation, and scientific applications. While there's no single answer, understanding sulfur's various isotopes and their abundance allows us to appreciate the complexity of this essential element and its crucial role in various aspects of our world. The study of sulfur and its isotopes continues to provide valuable insights across numerous scientific disciplines, highlighting the power of isotopic analysis in unraveling the mysteries of the natural world and advancing technological innovations. The next time you encounter the element sulfur, remember the intricate story contained within its atomic nucleus—a story written in protons, neutrons, and the variations that define its isotopic identity.
Latest Posts
Latest Posts
-
Are Daughter Cells Identical To Parent Cells In Mitosis
Apr 01, 2025
-
Hair Like Outgrowths That Attach To Bacteria
Apr 01, 2025
-
Which Of The Following Is An Example Of Ottonian Architecture
Apr 01, 2025
-
Antimicrobial Sensitivity Testing The Kirby Bauer Method
Apr 01, 2025
-
Calculate Ph Of A Weak Base
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Sulfur Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
