Calculate Ph Of A Weak Base
Muz Play
Apr 01, 2025 · 5 min read
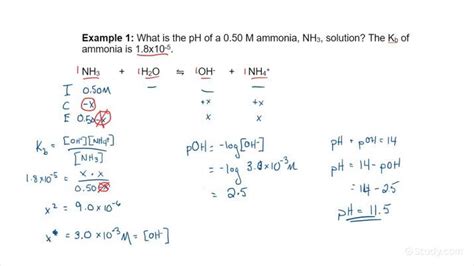
Table of Contents
Calculating the pH of a Weak Base: A Comprehensive Guide
Understanding how to calculate the pH of a weak base is crucial in various fields, including chemistry, environmental science, and biology. Unlike strong bases which completely dissociate in water, weak bases only partially ionize, leading to a more complex calculation. This comprehensive guide will walk you through the process, explaining the underlying concepts and providing step-by-step examples.
Understanding Weak Bases and Their Dissociation
A weak base is a substance that partially ionizes in water, meaning it doesn't completely break down into its constituent ions. This incomplete ionization is represented by an equilibrium reaction. Unlike strong bases like NaOH (sodium hydroxide) which dissociate completely, weak bases establish an equilibrium between the undissociated base and its ions.
Consider a generic weak base, B. Its dissociation in water can be represented as:
B(aq) + H₂O(l) ⇌ BH⁺(aq) + OH⁻(aq)
The equilibrium constant for this reaction is the base dissociation constant, denoted as K<sub>b</sub>. A smaller K<sub>b</sub> value indicates a weaker base. The expression for K<sub>b</sub> is:
Kb = [BH⁺][OH⁻] / [B]
where:
- [BH⁺] is the concentration of the conjugate acid
- [OH⁻] is the concentration of hydroxide ions
- [B] is the concentration of the undissociated weak base
The ICE Table Method: A Step-by-Step Approach
The ICE (Initial, Change, Equilibrium) table is a powerful tool for solving equilibrium problems, including calculating the pH of a weak base. Let's illustrate this with an example:
Example: Calculate the pH of a 0.10 M solution of ammonia (NH₃), a weak base with a K<sub>b</sub> of 1.8 x 10⁻⁵.
Step 1: Write the equilibrium reaction and the K<sub>b</sub> expression.
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
Kb = [NH₄⁺][OH⁻] / [NH₃] = 1.8 x 10⁻⁵
Step 2: Create the ICE table.
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| NH₃ | 0.10 | -x | 0.10 - x |
| NH₄⁺ | 0 | +x | x |
| OH⁻ | 0 | +x | x |
Step 3: Substitute the equilibrium concentrations into the K<sub>b</sub> expression.
Kb = (x)(x) / (0.10 - x) = 1.8 x 10⁻⁵
Step 4: Solve for x.
Since K<sub>b</sub> is small, we can often make the simplifying assumption that x is negligible compared to 0.10. This simplifies the equation to:
x² / 0.10 = 1.8 x 10⁻⁵
Solving for x:
x² = 1.8 x 10⁻⁶
x = √(1.8 x 10⁻⁶) = 1.34 x 10⁻³ M
This x value represents the equilibrium concentration of [OH⁻].
Step 5: Calculate the pOH.
pOH = -log[OH⁻] = -log(1.34 x 10⁻³) ≈ 2.87
Step 6: Calculate the pH.
Since pH + pOH = 14 at 25°C:
pH = 14 - pOH = 14 - 2.87 ≈ 11.13
Therefore, the pH of a 0.10 M ammonia solution is approximately 11.13.
When the Simplification is Not Valid
The simplification used in the previous example (ignoring x in the denominator) is only valid when K<sub>b</sub> is very small and the initial concentration of the base is relatively large. If this assumption is not valid, you need to solve the quadratic equation directly. This often involves using the quadratic formula:
x = [-b ± √(b² - 4ac)] / 2a
where the equation is in the form ax² + bx + c = 0.
Dealing with Polyprotic Weak Bases
Polyprotic weak bases can donate more than one proton. Calculating the pH of these bases requires considering multiple equilibrium reactions and their respective K<sub>b</sub> values. The calculations become more complex, but the fundamental principles remain the same – using ICE tables and the K<sub>b</sub> expressions for each dissociation step. Usually, the first dissociation step is the most significant in determining the overall pH.
Factors Affecting pH of Weak Bases
Several factors can influence the pH of a weak base solution:
- Concentration of the base: A higher concentration leads to a higher pH.
- Strength of the base (K<sub>b</sub>): A stronger base (larger K<sub>b</sub>) results in a higher pH.
- Temperature: K<sub>b</sub> values are temperature-dependent; changes in temperature affect the pH.
- Presence of common ions: The common ion effect can suppress the ionization of a weak base, lowering its pH.
Applications of Weak Base pH Calculations
Calculating the pH of weak bases has numerous applications:
- Environmental Science: Determining the pH of natural waters, assessing the impact of pollutants, and monitoring water quality.
- Chemistry: Understanding buffer solutions, acid-base titrations, and equilibrium processes.
- Biology: Studying biological systems where weak bases play a vital role, such as in blood pH regulation.
- Pharmacology: Analyzing the properties of pharmaceutical drugs and their interaction with biological systems.
Advanced Techniques and Considerations
For more complex scenarios, such as solutions containing mixtures of weak acids and weak bases or those involving polyprotic weak bases with significantly different K<sub>b</sub> values, more sophisticated methods may be necessary. These can include iterative numerical methods or using specialized software for equilibrium calculations. Understanding activity coefficients and their impact on equilibrium constants also becomes important at higher concentrations.
Conclusion
Calculating the pH of a weak base requires a thorough understanding of equilibrium chemistry and the use of tools like the ICE table. While the basic principles are relatively straightforward, more complex scenarios demand a deeper understanding of equilibrium calculations and might require more advanced techniques. Mastering these calculations is essential for success in various scientific and technical fields. Remember to always carefully consider the validity of simplifying assumptions and use appropriate methods to ensure accurate pH calculations.
Latest Posts
Latest Posts
-
Thesis Statement For Narrative Essay Example
Apr 02, 2025
-
Inborn Nonspecific Defenses Include And Barriers
Apr 02, 2025
-
Jewish Murals From The First Century Ce Depict
Apr 02, 2025
-
Which Quadratic Function Is Represented By The Graph
Apr 02, 2025
-
An Organized Arrangement Of Elements According To Their Atomic Number
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Calculate Ph Of A Weak Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
