A Nucleotide Triphosphate Has ___ Phosphate Groups.
Muz Play
Mar 16, 2025 · 7 min read
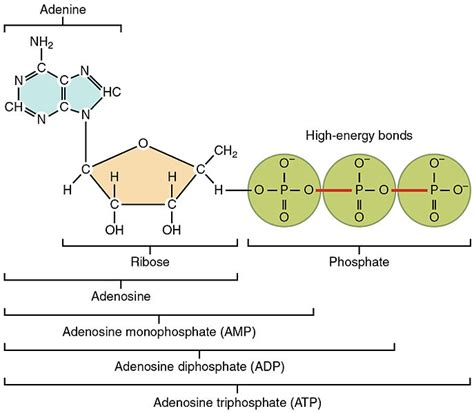
Table of Contents
A Nucleotide Triphosphate Has Three Phosphate Groups: Structure, Function, and Significance
A nucleotide triphosphate (NTP) is a fundamental molecule in biochemistry, serving as the building block for nucleic acids (DNA and RNA) and playing crucial roles in various cellular processes. The defining characteristic of an NTP, as the title suggests, is its possession of three phosphate groups. This seemingly simple structural feature is the key to understanding its remarkable versatility and importance in life. This article will delve deep into the structure, function, and significance of nucleotide triphosphates, focusing on the crucial role of those three phosphate groups.
Understanding the Structure of a Nucleotide Triphosphate
A nucleotide triphosphate is essentially a nucleotide with three phosphate groups attached. Let's break down this structure:
1. The Nitrogenous Base:
This is the first component of the nucleotide, and it's a nitrogen-containing ring structure. There are five main nitrogenous bases: adenine (A), guanine (G), cytosine (C), thymine (T) (found in DNA), and uracil (U) (found in RNA). The specific nitrogenous base determines the type of NTP (e.g., ATP, GTP, CTP, TTP, UTP).
2. The Pentose Sugar:
The second component is a five-carbon sugar. In RNA, this is ribose, while in DNA it's deoxyribose (lacking one oxygen atom). This seemingly small difference has significant implications for the stability and function of the nucleic acids.
3. The Triphosphate Group:
This is where the magic happens. The three phosphate groups are linked together by high-energy phosphoanhydride bonds. These bonds are crucial because their hydrolysis (breaking the bond with water) releases a significant amount of energy, which is harnessed to drive various cellular processes. This energy is often referred to as "high-energy phosphate bonds," although the term is somewhat of a simplification. The energy is not inherently stored in the phosphate bond itself, but rather in the difference in free energy between the reactants and products of hydrolysis. This energy difference is substantial, making NTPs excellent energy currency within cells. The three phosphate groups are typically denoted as α (alpha), β (beta), and γ (gamma), starting from the sugar.
In summary: A nucleotide triphosphate is composed of a nitrogenous base, a pentose sugar (ribose or deoxyribose), and a chain of three phosphate groups linked by high-energy phosphoanhydride bonds. It's this triphosphate group that distinguishes NTPs from nucleosides (base + sugar) and nucleoside monophosphates (base + sugar + one phosphate group).
The Significance of the Three Phosphate Groups
The presence of three phosphate groups is not merely a structural detail; it's fundamental to the NTP's function. Here's a breakdown:
1. Energy Currency:
The high-energy phosphoanhydride bonds between the phosphate groups are the primary reason NTPs serve as the cell's primary energy currency. The hydrolysis of these bonds, particularly the terminal γ-phosphate bond, releases a considerable amount of free energy, which can be coupled to drive energetically unfavorable reactions. This is essential for many cellular processes, including:
- Muscle contraction: ATP hydrolysis fuels the interactions between actin and myosin filaments, resulting in muscle movement.
- Active transport: The movement of molecules against their concentration gradient across cell membranes requires the energy from ATP hydrolysis.
- Biosynthesis: The synthesis of macromolecules like proteins, nucleic acids, and polysaccharides is energy-intensive and relies heavily on ATP and other NTPs.
- Signal transduction: Many cellular signaling pathways involve the phosphorylation of proteins by kinases, a process powered by NTPs.
2. Building Blocks of Nucleic Acids:
NTPs are the precursors for the synthesis of DNA and RNA. During DNA replication and RNA transcription, the nucleoside triphosphates (dNTPs for DNA and NTPs for RNA) are incorporated into the growing nucleic acid chain. The hydrolysis of the two terminal phosphate groups (β and γ) provides the energy to drive the polymerization reaction, forming the phosphodiester bonds that link the nucleotides together.
3. Cofactors in Enzymatic Reactions:
Besides their roles in energy transfer and nucleic acid synthesis, NTPs also serve as cofactors in various enzymatic reactions. Some enzymes require NTPs to bind to their active sites, inducing conformational changes that facilitate catalysis. This is particularly true for enzymes involved in signal transduction and metabolic pathways.
4. Regulation of Cellular Processes:
The concentrations of various NTPs within a cell act as important signaling molecules. Changes in NTP levels can influence gene expression, metabolic pathways, and other cellular processes. For example, the ratio of ATP to ADP serves as an indicator of the cell's energy status.
Specific Examples of Nucleotide Triphosphates
Let's examine some key NTPs and their specific roles:
1. Adenosine Triphosphate (ATP):
ATP is undoubtedly the most ubiquitous and important NTP. It's often called the "energy currency" of the cell because of its central role in energy transfer. ATP hydrolysis fuels a vast array of cellular processes, as detailed above.
2. Guanosine Triphosphate (GTP):
GTP plays a vital role in protein synthesis (translation), acting as an energy source for the translocation of the ribosome along the mRNA molecule. It's also crucial in signal transduction pathways, particularly those involving G proteins.
3. Cytidine Triphosphate (CTP):
CTP is primarily involved in lipid synthesis, serving as a substrate for the formation of phospholipids, the major components of cell membranes.
4. Uridine Triphosphate (UTP):
UTP participates in carbohydrate metabolism, being a crucial component in the synthesis of glycogen, the storage form of glucose in animals.
5. Thymidine Triphosphate (TTP):
Exclusively found in DNA synthesis, TTP is one of the four dNTPs necessary for DNA replication. It's incorporated opposite to adenine during DNA replication.
The Hydrolysis of Phosphate Bonds: The Energy Source
The high-energy phosphoanhydride bonds in NTPs are the source of the energy they provide. When these bonds are hydrolyzed (broken down by water), a substantial amount of free energy is released. This energy is not stored in the bond itself, but rather arises from the difference in free energy between the reactants (NTP) and the products (NDP + Pi, where NDP is nucleoside diphosphate and Pi is inorganic phosphate). This energy difference is exploited by cells to drive various endergonic reactions (reactions requiring energy input).
The hydrolysis of ATP, for instance, is a highly exergonic reaction, releasing approximately 30.5 kJ/mol of energy under standard conditions. This released energy can be harnessed by coupling it to an endergonic reaction, making the overall process thermodynamically favorable. This coupling often involves the transfer of a phosphate group to another molecule, a process known as phosphorylation. Phosphorylation can alter the conformation and activity of proteins, impacting their function.
Maintaining NTP Balance: A Cellular Balancing Act
Maintaining the appropriate balance of NTPs within a cell is crucial for its proper functioning. The cell employs various mechanisms to regulate NTP levels, including:
- Substrate availability: The availability of precursors for NTP synthesis (e.g., amino acids, ribose-5-phosphate) directly influences the rate of NTP production.
- Enzyme regulation: The activities of enzymes involved in NTP synthesis and degradation are carefully regulated, often through allosteric mechanisms (changes in enzyme conformation in response to the binding of a molecule) or feedback inhibition (the end-product of a metabolic pathway inhibits an enzyme earlier in the pathway).
- Compartmentalization: NTPs are not uniformly distributed throughout the cell but are often compartmentalized within specific organelles, such as mitochondria (the powerhouse of the cell), to optimize their utilization.
Conclusion: The Indispensable Role of Nucleotide Triphosphates
Nucleotide triphosphates are essential molecules in all living organisms. Their three phosphate groups are fundamental to their function, providing the high-energy bonds necessary for energy transfer and serving as building blocks for nucleic acids. Their roles extend far beyond energy currency and encompass participation in numerous metabolic and signaling pathways. Understanding the structure and function of NTPs is crucial to comprehending the fundamental principles of cellular biochemistry and the intricate mechanisms that govern life. The precise balance of different NTPs and the regulatory mechanisms that maintain this balance are vital to cell homeostasis and proper biological function. Further research continually unveils the multifaceted roles of these remarkable molecules in various cellular processes, highlighting their continued significance in biological research.
Latest Posts
Latest Posts
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
-
Electric Potential From A Point Charge
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about A Nucleotide Triphosphate Has ___ Phosphate Groups. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
