According To Dalton Atoms Of Different Elements Will Be
Muz Play
Mar 17, 2025 · 6 min read
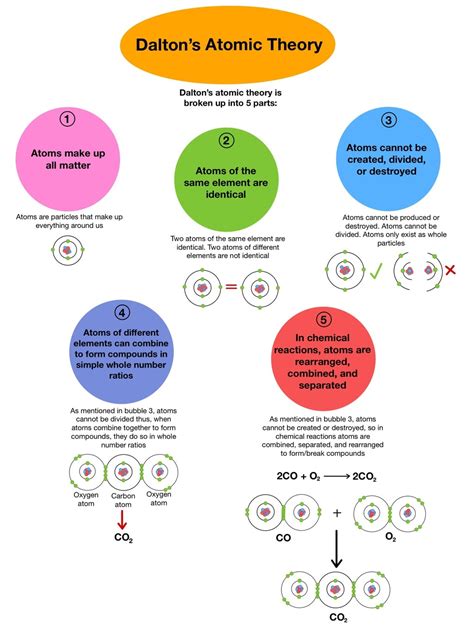
Table of Contents
According to Dalton: Atoms of Different Elements Will Be... Different! Unveiling the Foundation of Modern Chemistry
John Dalton's atomic theory, proposed in the early 1800s, revolutionized our understanding of matter. While some aspects have been refined or superseded by modern quantum mechanics, its core tenets remain foundational to chemistry. One of the most crucial aspects of Dalton's theory is his postulate regarding the nature of atoms from different elements: atoms of different elements will be different. This seemingly simple statement laid the groundwork for our current understanding of the periodic table, chemical reactions, and the very building blocks of the universe. Let's delve deeper into this pivotal concept and explore its implications.
Dalton's Atomic Theory: A Cornerstone of Chemistry
Before diving into the specifics of atomic differences, let's briefly review the key postulates of Dalton's atomic theory:
-
All matter is made of atoms: This fundamental assertion established the atom as the basic unit of matter. While we now know atoms are composed of subatomic particles, this initial proposition was groundbreaking.
-
Atoms are indivisible and indestructible: Dalton initially believed atoms were solid, indivisible spheres. This notion has been modified with the discovery of subatomic particles, but the concept of atoms being the fundamental units in chemical reactions largely holds true.
-
All atoms of a given element are identical in mass and properties: This is where the focus of our discussion begins. Dalton proposed that all atoms of a specific element – such as oxygen or hydrogen – possess identical mass and properties. This concept is crucial for understanding the consistent behavior of elements in chemical reactions.
-
Atoms of different elements differ in mass and properties: This is the key postulate for this article. Dalton asserted that atoms of different elements are fundamentally distinct in their mass and properties. This distinction is what allows for the variety of substances we observe in the world around us.
-
Atoms combine in simple, whole-number ratios to form compounds: This postulate explains the law of definite proportions, stating that a compound always contains the same elements in the same proportion by mass. It underscores the discrete nature of atoms and their role in forming molecules.
The Significance of Atomic Differences: Beyond Mass and Properties
Dalton's assertion that atoms of different elements are different goes beyond simply stating they have different masses. The differences encompass a range of properties that govern their chemical behavior. These properties include:
-
Atomic Mass: This is perhaps the most obvious difference. Different elements have different numbers of protons and neutrons in their nuclei, leading to variations in their atomic mass. This difference is crucial in determining the relative quantities of elements in a chemical reaction, dictated by stoichiometry.
-
Atomic Number: The atomic number, representing the number of protons in an atom's nucleus, uniquely identifies an element. This number defines the element's position on the periodic table and dictates its chemical properties. It's the fundamental characteristic distinguishing one element from another.
-
Electron Configuration: The arrangement of electrons in an atom's electron shells profoundly influences its chemical behavior. Elements with similar electron configurations often exhibit similar chemical properties, a pattern clearly reflected in the periodic table's organization.
-
Electronegativity: This property reflects an atom's tendency to attract electrons within a chemical bond. Differences in electronegativity between atoms determine the type of bond formed (ionic, covalent, metallic) and the properties of the resulting compound.
-
Ionization Energy: The energy required to remove an electron from an atom is another crucial property that varies significantly among elements. This difference influences an element's reactivity and its tendency to form ions.
-
Reactivity: The overall chemical reactivity of an element is a direct consequence of its atomic structure and its various properties. Highly reactive elements readily participate in chemical reactions, while less reactive elements are more stable.
Implications of Atomic Differences: Understanding Chemical Reactions
The differences between atoms of different elements are the driving force behind chemical reactions. Chemical reactions involve the rearrangement of atoms to form new substances. These rearrangements are governed by the unique properties of the atoms involved.
For example, consider the reaction between sodium (Na) and chlorine (Cl) to form sodium chloride (NaCl), common table salt. Sodium has a low ionization energy and readily loses an electron to form a positively charged ion (Na+). Chlorine, with high electronegativity, readily gains an electron to form a negatively charged ion (Cl-). The electrostatic attraction between these oppositely charged ions forms the ionic bond in NaCl. This simple example highlights how the distinct properties of sodium and chlorine atoms dictate their interaction and the formation of a new compound with entirely different properties.
This principle extends to all chemical reactions. The specific properties of the reacting atoms—their sizes, electronegativities, ionization energies, and electron configurations—determine the feasibility and nature of the reaction. Understanding these differences is essential for predicting reaction outcomes and manipulating chemical processes.
The Periodic Table: A Manifestation of Atomic Differences
The periodic table is a direct testament to the differences between atoms of different elements. The table organizes elements based on their increasing atomic number, reflecting the systematic variation in their atomic structure and properties. Elements within the same group (vertical column) share similar chemical properties due to their similar electron configurations in their outermost shell (valence electrons). Elements in the same period (horizontal row) exhibit a gradual change in properties as the atomic number increases, reflecting the filling of electron shells.
The periodic table provides a powerful tool for predicting the behavior of elements and understanding the relationships between them. It’s a visual representation of Dalton's fundamental concept – the inherent differences between atoms of different elements.
Beyond Dalton: Modern Refinements and Quantum Mechanics
While Dalton's atomic theory was revolutionary, some aspects have been refined by subsequent discoveries. The discovery of subatomic particles—protons, neutrons, and electrons—demonstrated that atoms are not indivisible. Furthermore, isotopes of the same element exist, possessing the same number of protons but differing numbers of neutrons, resulting in variations in atomic mass within an element.
Quantum mechanics provides a far more sophisticated and accurate description of atomic structure and behavior. It explains the wave-particle duality of electrons, the quantization of energy levels, and the probability distributions of electrons within atoms. This advanced understanding refines our comprehension of atomic properties and chemical bonding but doesn't negate the fundamental principle established by Dalton: atoms of different elements possess distinct properties.
Conclusion: The Enduring Legacy of Dalton's Insight
Despite the advancements in atomic theory since Dalton's time, his assertion that atoms of different elements are different remains a cornerstone of modern chemistry. This fundamental understanding underpins our knowledge of the periodic table, chemical reactions, and the vast diversity of substances found in the universe. Dalton's contribution laid the foundation for centuries of scientific progress, demonstrating the power of insightful observation and the enduring impact of a simple, yet profound, idea. The differences between atoms, as highlighted by Dalton, remain the key to unlocking the secrets of the chemical world. From the simplest molecules to the most complex biological systems, the distinct characteristics of atoms of different elements are the fundamental building blocks of all matter, driving its behavior and shaping the world around us. Further explorations into the subtle intricacies of atomic interactions continue to unveil the complexities and wonders of the chemical universe. Understanding these differences is not just a cornerstone of chemistry; it’s the key to unlocking the secrets of the material world and its diverse interactions.
Latest Posts
Latest Posts
-
Atoms In Molecules Share Pairs Of Electrons When They Make
Mar 17, 2025
-
Exponential And Logarithmic Functions Examples With Solutions
Mar 17, 2025
-
Graph That Does Not Represent A Function
Mar 17, 2025
-
Animals Store Energy In The Form Of
Mar 17, 2025
-
Enzymes Decrease The Activation Energy Of A Reaction By
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about According To Dalton Atoms Of Different Elements Will Be . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
