Are Strong Bases Good Leaving Groups
Muz Play
Mar 17, 2025 · 5 min read
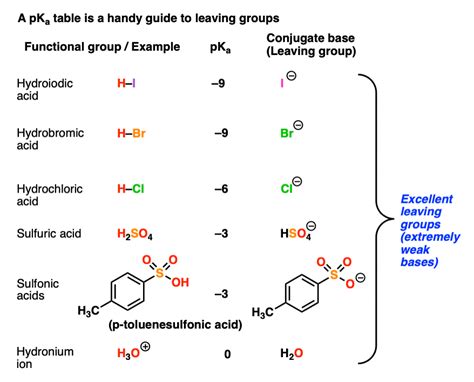
Table of Contents
Are Strong Bases Good Leaving Groups? A Deep Dive into Reaction Mechanisms
The question of whether strong bases make good leaving groups is a nuanced one, often leading to confusion in organic chemistry. The simple answer is: generally no, strong bases are poor leaving groups. However, understanding why requires a deeper exploration of reaction mechanisms, stability, and the interplay of various factors influencing reaction kinetics and thermodynamics. This article will delve into the intricacies of leaving group ability, contrasting strong bases with good leaving groups, and exploring exceptions to the rule.
Understanding Leaving Groups: A Fundamental Concept
Leaving groups (LG) are crucial in many organic reactions, particularly nucleophilic substitution (SN1 and SN2) and elimination (E1 and E2) reactions. A good leaving group is one that can readily depart from a molecule, taking a pair of electrons with it to form a stable species. The stability of the departing species is directly correlated to its leaving group ability. The better the leaving group, the faster the reaction will proceed.
Key Characteristics of Good Leaving Groups:
- Weak Conjugate Base: Good leaving groups are weak bases. This means their conjugate acids are strong acids. A weak base is less likely to re-bond with the molecule it left, facilitating the reaction's progression.
- Stability: The departed species should be stable and capable of independently existing as an anion or neutral molecule. Resonance stabilization, inductive effects, and high electronegativity significantly contribute to stability.
- Polarizability: A highly polarizable leaving group can better disperse the negative charge it acquires upon departure, thus stabilizing the transition state and accelerating the reaction.
Strong Bases: The Opposite of Good Leaving Groups
Strong bases, by definition, have a strong affinity for protons (H⁺). This inherent property directly conflicts with the characteristics of a good leaving group. Let's consider why:
- High Basicity Implies Poor Leaving Ability: A strong base readily accepts a proton. This means it's reluctant to depart and leave behind a negative charge. If it did leave, it would immediately seek a proton to neutralize itself. This severely hinders the reaction.
- Instability of the Conjugate Acid: The conjugate acid of a strong base is a weak acid. This weak conjugate acid lacks the stability needed for a species to easily detach from the molecule.
- Reversibility of Reactions: The strong base's tendency to re-protonate and re-attach to the molecule can result in reversible reactions, preventing the formation of desired products.
Examples of Strong Bases and Their Poor Leaving Group Behavior:
- Hydroxide Ion (OH⁻): A very strong base, OH⁻ is a poor leaving group in most reactions. Reactions involving OH⁻ as a leaving group often require harsh conditions or specific catalytic strategies.
- Alkoxide Ions (RO⁻): Similar to OH⁻, alkoxide ions are strong bases and are generally poor leaving groups. Their conjugate acids (alcohols) are relatively weak.
- Amide Ion (NH₂⁻): An extremely strong base, amide ion is an exceptionally poor leaving group.
Exceptions and Nuances: When Strong Bases Might Seem to Leave
While the general rule holds true, there are specific circumstances where a species often considered a strong base might appear to act as a leaving group. However, it's crucial to understand that these situations usually involve complex mechanisms and don't contradict the fundamental principles discussed earlier.
1. Elimination Reactions (E2):
In E2 reactions, a strong base abstracts a proton while simultaneously eliminating another group. While the strong base isn't leaving as a leaving group in the traditional sense, it's involved in the elimination process. The leaving group in an E2 reaction is usually a halide or a tosylate. The strong base facilitates the elimination, but it isn't the leaving group itself.
2. Internal Displacement Reactions:
In intramolecular reactions, a strong base within a molecule might participate in an internal displacement or cyclization. While it starts as a part of the molecule, it ultimately ends up as a separate fragment, creating the impression of a strong base acting as a leaving group. This is driven by the formation of a stable ring structure or a more stable molecule. However, it's critical to recognize the mechanistic difference from typical SN1 or SN2 reactions.
3. Specific Catalytic Systems:
Certain catalytic systems can facilitate reactions where strong bases are involved in what might seem like a leaving group process. These systems often utilize transition metals or other catalysts to stabilize intermediates and facilitate otherwise unfavorable reactions. The catalyst plays a critical role in stabilizing the strong base and allowing it to participate in a way that wouldn't be possible under standard conditions.
Comparing Strong Bases to Good Leaving Groups: A Table
| Feature | Strong Base | Good Leaving Group |
|---|---|---|
| Basicity | High | Low |
| Conjugate Acid | Weak acid | Strong acid |
| Stability | Often unstable as an anion | Stable as an anion or neutral molecule |
| Leaving Tendency | Reluctant to leave; readily accepts a proton | Readily leaves; less likely to re-bond |
| Examples | OH⁻, RO⁻, NH₂⁻, R₂N⁻ | I⁻, Br⁻, Cl⁻, TsO⁻, H₂O, RCOOH |
Improving Leaving Group Ability of Weak Bases
If a molecule contains a poor leaving group (such as hydroxide), it's often necessary to transform it into a better leaving group before carrying out a reaction. This is commonly achieved by:
- Protonation: Protonating a hydroxyl group (–OH) converts it to water (H₂O), a much better leaving group. Strong acids like H₂SO₄ are used for this purpose.
- Conversion to Tosylates or Mesylates: Converting alcohols or other weak bases into tosylates (–OTs) or mesylates (–OMs) significantly enhances their leaving group ability due to resonance stabilization of the leaving group.
- Formation of Activated Esters: Similar to tosylates, the formation of activated esters improves leaving group ability through resonance stabilization.
Conclusion: Context Matters
While the general rule remains that strong bases are poor leaving groups, the complexities of reaction mechanisms and the potential influence of catalysts and specific reaction conditions mean that exceptions can exist. A thorough understanding of reaction mechanisms, the stability of involved species, and the impact of catalysis is vital for accurately predicting reaction outcomes and designing synthetic strategies. Remember to always analyze the specific reaction context to determine the true behavior of a potential leaving group. Focusing on the stability of the leaving group and its conjugate acid is essential for predicting the reaction's feasibility and speed.
Latest Posts
Latest Posts
-
Does Water Go From High To Low Concentration
Mar 17, 2025
-
Circle Math Triangle Extending From Circle
Mar 17, 2025
-
According To The Kinetic Theory Of Gases
Mar 17, 2025
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Are Strong Bases Good Leaving Groups . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
