A Solution Is Composed Of A Dissolved In A
Muz Play
Mar 16, 2025 · 6 min read
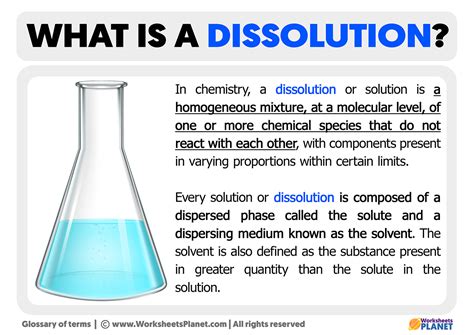
Table of Contents
A Solution: Understanding Solutes, Solvents, and the Dissolution Process
A solution, in its simplest form, is a homogeneous mixture composed of two or more substances. This seemingly straightforward definition belies a rich and complex world of interactions at the molecular level. Understanding solutions is crucial across numerous scientific disciplines, from chemistry and biology to materials science and environmental studies. This in-depth exploration delves into the fundamental components of a solution – the solute dissolved in a solvent – and the fascinating processes that govern their interaction.
Defining the Components: Solute and Solvent
Before we delve into the intricacies of solution formation, let's clarify the key terms:
Solute: The Dissolved Substance
The solute is the substance that dissolves in a solution. It's typically present in a smaller amount compared to the solvent. Solutes can be solids, liquids, or gases. For example, in a saltwater solution, salt (NaCl) is the solute. The properties of the solute significantly influence the properties of the resulting solution, such as its color, conductivity, and boiling point.
Examples of Solutes:
- Solids: Sugar, salt, copper sulfate
- Liquids: Ethanol, acetic acid
- Gases: Carbon dioxide, oxygen
Solvent: The Dissolving Medium
The solvent is the substance that dissolves the solute. It's usually the component present in the larger amount. Water is often referred to as the "universal solvent" due to its exceptional ability to dissolve a wide range of substances. However, other solvents exist, each with its own unique dissolving capabilities. The choice of solvent is critical in determining the solubility of a particular solute.
Examples of Solvents:
- Water (H₂O): The most common solvent, polar in nature.
- Ethanol (C₂H₅OH): A polar organic solvent, often used in alcoholic beverages and various industrial processes.
- Acetone (CH₃COCH₃): A nonpolar organic solvent, commonly used in nail polish remover and cleaning solutions.
The Dissolution Process: A Molecular Perspective
The process of dissolution is far from passive; it's a dynamic interplay between solute and solvent molecules governed by intermolecular forces. Let's examine the steps involved:
-
Solvent Attack: The solvent molecules approach the solute particles (ions or molecules). The solvent molecules must have a sufficient attraction to the solute particles to overcome the attractive forces holding the solute together. This is crucial for the dissolution process to occur.
-
Breaking Intermolecular Forces: The solvent molecules exert forces on the solute particles, weakening and ultimately breaking the intermolecular forces (e.g., ionic bonds, hydrogen bonds, van der Waals forces) holding the solute together. This requires energy input, often in the form of heat. The energy required to overcome these forces is called the lattice energy for solids, and it is an important factor in determining the solubility of the solute.
-
Solvation: Once the solute particles are separated, they become surrounded by solvent molecules. This process is known as solvation. If the solvent is water, the process is called hydration. This solvation shell shields the solute particles from each other, preventing them from re-aggregating. The strength of the solute-solvent interactions dictates the effectiveness of solvation.
-
Diffusion: The dissolved solute particles distribute evenly throughout the solvent through a process called diffusion. This ensures a homogeneous mixture, characteristic of a true solution.
Factors Affecting Solubility
Several factors influence the solubility of a solute in a given solvent:
1. Nature of the Solute and Solvent: "Like Dissolves Like"
The fundamental principle governing solubility is the "like dissolves like" rule. Polar solvents tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. This is because polar molecules have positive and negative regions, allowing them to interact strongly with other polar molecules through dipole-dipole interactions and hydrogen bonds. Similarly, nonpolar molecules interact primarily through weak van der Waals forces.
Examples:
- Polar solute in polar solvent: Sugar (polar) dissolving in water (polar).
- Nonpolar solute in nonpolar solvent: Oil (nonpolar) dissolving in gasoline (nonpolar).
- Polar solute in nonpolar solvent: Salt (polar) will not dissolve in oil (nonpolar).
2. Temperature
The effect of temperature on solubility varies depending on whether the dissolution process is endothermic or exothermic.
-
Endothermic Dissolution: For most solid solutes dissolving in liquid solvents, the dissolution process is endothermic (absorbs heat). Increasing the temperature increases the solubility of the solute. The added heat provides the energy needed to break the solute-solute interactions.
-
Exothermic Dissolution: In some cases, dissolution is exothermic (releases heat). Increasing the temperature decreases the solubility. This is less common for solid solutes.
The solubility of gases in liquids generally decreases with increasing temperature. The increased kinetic energy of the gas molecules allows them to escape the solvent more easily.
3. Pressure
Pressure primarily affects the solubility of gases in liquids. According to Henry's Law, the solubility of a gas is directly proportional to the partial pressure of the gas above the liquid. Increasing the pressure increases the solubility of the gas. This is why carbonated beverages fizz more when the bottle is opened, releasing pressure and allowing the dissolved carbon dioxide to escape. The effect of pressure on the solubility of solids and liquids is negligible.
4. Particle Size
Smaller solute particles dissolve faster than larger ones. This is because a larger surface area is exposed to the solvent, providing more points of contact for interaction and dissolution. The solubility itself is not directly affected, but the rate of dissolution is significantly influenced.
Types of Solutions
Solutions are classified based on the relative amounts of solute and solvent:
- Dilute Solutions: Contain a relatively small amount of solute compared to the solvent.
- Concentrated Solutions: Contain a relatively large amount of solute.
- Saturated Solutions: Contain the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. Adding more solute will not result in further dissolution; it will simply precipitate out.
- Unsaturated Solutions: Contain less solute than the maximum amount that can dissolve. More solute can be added and dissolved.
- Supersaturated Solutions: Contain more solute than a saturated solution can normally hold. These are unstable and can be easily precipitated out by adding a seed crystal or disturbing the solution.
Applications of Solutions
The importance of solutions extends across diverse fields:
-
Medicine: Many medications are administered in solution form for easy absorption by the body. Intravenous fluids are solutions carefully formulated to maintain electrolyte balance.
-
Agriculture: Fertilizers are often applied as solutions to ensure efficient nutrient uptake by plants.
-
Industry: A wide range of industrial processes rely on solutions, from cleaning and etching to chemical reactions and electroplating.
-
Environmental Science: Understanding the solubility of pollutants is crucial in assessing their impact on water bodies and ecosystems.
Conclusion
The seemingly simple concept of a solution – a solute dissolved in a solvent – encompasses a wealth of scientific principles and practical applications. From the molecular interactions driving dissolution to the factors influencing solubility, understanding solutions is fundamental to numerous scientific and technological advancements. The "like dissolves like" rule serves as a powerful guideline, while temperature, pressure, and particle size modulate the rate and extent of dissolution. The diverse applications of solutions highlight their pervasive importance in our daily lives and across various industries. Further exploration of specific solution types, their properties, and behaviors will continue to refine our understanding of this foundational chemical concept.
Latest Posts
Latest Posts
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
-
Electric Potential From A Point Charge
Mar 17, 2025
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about A Solution Is Composed Of A Dissolved In A . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
