A Supersaturated Solution Is One That
Muz Play
Mar 17, 2025 · 6 min read
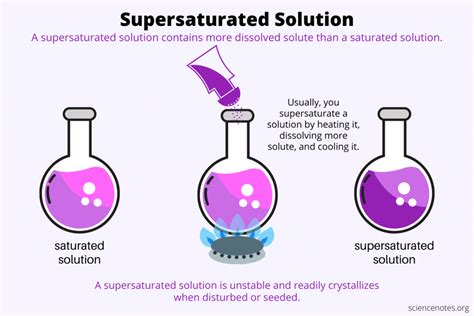
Table of Contents
A Supersaturated Solution Is One That… Holds More Than Its Fair Share!
A supersaturated solution is a fascinating chemical phenomenon that challenges our basic understanding of solubility. It's a solution that contains more dissolved solute than it theoretically should at a given temperature and pressure. Think of it as a delicate balancing act, where the solute is crammed into the solvent beyond its normal capacity. This seemingly unstable state is crucial in several industrial processes and offers a compelling window into the principles of thermodynamics and kinetics. Let's delve deeper into the intricacies of supersaturated solutions.
Understanding Solubility: The Foundation
Before exploring supersaturation, we need to grasp the concept of solubility. Solubility refers to the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure to form a stable solution. This is usually expressed as grams of solute per 100 grams of solvent or as molarity (moles of solute per liter of solution). For instance, sugar dissolves readily in water, while sand is largely insoluble. The solubility of a substance is influenced by factors like temperature, pressure (especially for gases), and the nature of both solute and solvent (polarity, intermolecular forces). A solution that has dissolved the maximum amount of solute at a given temperature and pressure is called a saturated solution. Any additional solute added to a saturated solution will simply settle at the bottom undissolved.
Saturation: A State of Equilibrium
A saturated solution exists in a state of dynamic equilibrium. At the microscopic level, solute particles are constantly dissolving from the solid phase and precipitating (coming out of solution) back into the solid phase at equal rates. This means the rate of dissolution equals the rate of precipitation, resulting in no net change in the concentration of the dissolved solute.
Supersaturation: Beyond the Limit
Now, let's return to our star player: the supersaturated solution. This is a solution that contains more solute than a saturated solution at the same temperature and pressure. It's a metastable state, meaning it's thermodynamically unstable but kinetically stable. This means it can exist for a period, but any minor disturbance can trigger the excess solute to precipitate out, returning the solution to a saturated state.
How is Supersaturation Achieved?
Creating a supersaturated solution requires careful manipulation of the system, often involving altering temperature or pressure. Here are some common methods:
-
Heating and Cooling: This is a widely used technique. The solubility of many solids increases with temperature. You dissolve a large amount of solute in a solvent at a high temperature. Then, the solution is carefully cooled slowly without disturbing it. If the cooling is slow enough, the excess solute may remain dissolved, resulting in a supersaturated solution. This method relies on the fact that the rate of crystallization is slower than the rate of cooling. However, any introduction of a seed crystal (a small crystal of the solute) or agitation can initiate rapid crystallization.
-
Evaporation: Another approach involves preparing a saturated solution and then carefully evaporating the solvent. As the solvent evaporates, the concentration of the solute increases, ultimately surpassing the solubility limit at the given temperature, leading to supersaturation. Similar to the heating and cooling method, the slightest disturbance can cause crystallization.
-
Chemical Reaction: Supersaturated solutions can also result from chemical reactions where a soluble product forms in excess of its solubility limit.
The Metastable Nature of Supersaturation
The key characteristic of a supersaturated solution is its metastability. It's a state of apparent stability, but it's easily disrupted. This instability arises from the higher energy state of the supersaturated solution compared to the saturated solution. The excess solute is essentially "holding on" in the solution, but it’s not thermodynamically favored.
Nucleation and Crystallization: The Breaking Point
The process of converting a supersaturated solution back to a saturated solution is initiated by nucleation. Nucleation is the formation of tiny solid particles (nuclei) of the solute within the solution. These nuclei provide a surface for further crystallization, which is the growth of larger crystals from the dissolved solute.
Several factors can trigger nucleation:
-
Introduction of seed crystals: As mentioned earlier, adding a tiny crystal of the solute acts as a nucleation site, rapidly initiating crystallization.
-
Scratching the container: Even a minor scratch on the container can create nucleation sites, leading to crystallization.
-
Agitation or shaking: Physical disturbance can provide the energy needed for nucleation to occur.
-
Changes in temperature or pressure: Fluctuations in temperature or pressure can disrupt the metastable equilibrium, initiating nucleation.
Once nucleation starts, crystallization proceeds rapidly until the solution becomes saturated and the equilibrium is re-established. The rate of crystallization depends on factors like the degree of supersaturation, temperature, and the presence of impurities.
Applications of Supersaturated Solutions
Supersaturated solutions, despite their apparent instability, find various applications across different industries. Here are some prominent examples:
1. Candy Making: Rock Candy and Beyond
The creation of rock candy is a classic example of supersaturation. A sugar solution is supersaturated, and then a string or stick is introduced to act as a nucleation site. Sugar crystals grow on this site, forming large, beautiful rock candy crystals.
2. Chemical Crystallization: High-Purity Crystals
In various chemical processes, supersaturated solutions are used to produce large, high-purity crystals. The controlled precipitation of solute from a supersaturated solution allows for the growth of crystals with well-defined structures and fewer imperfections. This is essential in applications requiring high-quality crystals, like in pharmaceuticals or semiconductors.
3. Medical Applications: Drug Delivery
Supersaturated solutions are being investigated for drug delivery systems. By increasing the solubility of poorly soluble drugs, it's possible to enhance the bioavailability and efficacy of medications. This offers the potential for improved treatment outcomes for various conditions.
4. Photography: Developing Processes
Certain photographic processes rely on the controlled precipitation of silver halide crystals from a supersaturated solution. The precise control over crystallization is crucial for obtaining high-quality images.
5. Geochemistry: Mineral Formation
Supersaturation plays a role in the formation of minerals in geological environments. When the concentration of dissolved ions exceeds the solubility limit, mineral precipitation occurs, leading to the formation of rocks and ores.
Distinguishing Between Different Types of Solutions
It's important to clearly differentiate between saturated, unsaturated, and supersaturated solutions:
-
Unsaturated solution: Contains less solute than it can dissolve at a given temperature and pressure. More solute can be dissolved without changing the solution's properties.
-
Saturated solution: Contains the maximum amount of solute that can dissolve at a given temperature and pressure. Adding more solute results in undissolved solute remaining.
-
Supersaturated solution: Contains more solute than it can normally dissolve at a given temperature and pressure. It's a metastable state and easily returns to a saturated state upon disturbance.
Conclusion: A Delicate Balance
Supersaturated solutions represent a fascinating area of chemistry where thermodynamics and kinetics interplay to create a metastable state. Their ability to hold more solute than theoretically possible offers opportunities for various applications, from candy making to advanced drug delivery systems. Understanding the principles of solubility, nucleation, and crystallization is crucial in controlling and utilizing the unique properties of supersaturated solutions. The delicate balance between dissolved solute and the potential for rapid precipitation underscores the complexity and elegance of solution chemistry. Further research into this area will undoubtedly unveil new and exciting applications in various scientific and industrial fields.
Latest Posts
Latest Posts
-
Using E Z Designators Identify The Configuration
Mar 17, 2025
-
Differences Between Eukaryotic And Prokaryotic Gene Expression
Mar 17, 2025
-
Understanding The Definitions Of Ionization Energy And Electron Affinity
Mar 17, 2025
-
The Sodium Potassium Pump Is An Example Of
Mar 17, 2025
-
Select All That Are Functions Of Proteins
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about A Supersaturated Solution Is One That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
