According To Arrhenius Theory What Is An Acid
Muz Play
Mar 17, 2025 · 5 min read
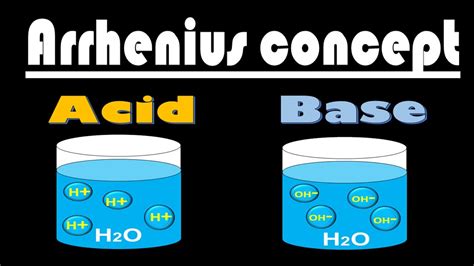
Table of Contents
According to Arrhenius Theory: What is an Acid?
The world of chemistry is filled with fascinating concepts, and one of the foundational ideas is the understanding of acids and bases. While numerous theories exist to explain acid-base behavior, the Arrhenius theory, proposed by Svante Arrhenius in 1884, remains a crucial starting point for grasping the fundamental nature of acids. This theory, while having limitations, provides a clear and concise definition that forms the basis for more advanced models. This article will delve deep into the Arrhenius definition of an acid, exploring its implications, limitations, and its continuing relevance in chemistry.
The Arrhenius Definition: A Simple Yet Powerful Concept
According to Arrhenius's theory, an acid is any substance that, when dissolved in water, increases the concentration of hydrogen ions (H⁺). This seemingly simple definition has profound implications for understanding chemical reactions and the properties of acidic solutions. The key here is the presence of water as the solvent. The dissociation of the acid in water is crucial for the generation of hydrogen ions, which are responsible for the characteristic properties of acids.
Let's consider a common example: hydrochloric acid (HCl). When HCl is dissolved in water, it undergoes complete dissociation, breaking apart into its constituent ions:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
The resulting solution contains a high concentration of hydrogen ions (H⁺), making it acidic. This increase in H⁺ concentration is the defining characteristic of an Arrhenius acid. The chloride ions (Cl⁻) are also present, but they don't directly contribute to the acidic properties of the solution. It’s the abundance of H⁺ that determines the acidity.
Understanding Hydrogen Ions (H⁺) and Hydronium Ions (H₃O⁺)
It's important to clarify a common misconception regarding hydrogen ions. In reality, free, unassociated protons (H⁺) are rarely found in aqueous solutions. The highly charged proton readily interacts with water molecules, forming a hydronium ion (H₃O⁺).
The reaction is:
H⁺(aq) + H₂O(l) → H₃O⁺(aq)
Therefore, while Arrhenius defined acids based on the production of H⁺, it's more accurate to say that they increase the concentration of hydronium ions (H₃O⁺) in aqueous solution. However, the terms H⁺ and H₃O⁺ are often used interchangeably, with the understanding that the latter is a more precise representation of the species present in solution.
Strong Acids vs. Weak Acids: A Matter of Degree
The Arrhenius theory also helps categorize acids based on their degree of dissociation in water. Strong acids are those that completely dissociate into their ions in aqueous solution. Examples include HCl (hydrochloric acid), HBr (hydrobromic acid), HI (hydroiodic acid), HNO₃ (nitric acid), H₂SO₄ (sulfuric acid), and HClO₄ (perchloric acid). These acids essentially release all their hydrogen ions in water.
Weak acids, on the other hand, only partially dissociate in water. This means that only a small fraction of the acid molecules break down into ions, leaving a significant portion in their undissociated form. Examples include acetic acid (CH₃COOH), formic acid (HCOOH), and carbonic acid (H₂CO₃). The equilibrium between the undissociated acid and its ions is crucial in understanding the behavior of weak acids. This equilibrium can be expressed using an equilibrium constant, known as the acid dissociation constant (Ka). A smaller Ka value indicates a weaker acid.
The Role of Hydrogen Ions in Acidic Properties
The increased concentration of hydrogen ions (or hydronium ions) is responsible for the characteristic properties associated with acids:
-
Sour taste: Many acids have a characteristic sour taste, though it's extremely dangerous to taste unknown chemicals to test for acidity. This taste is directly linked to the interaction of H⁺ ions with taste receptors.
-
Reaction with metals: Acids react with many metals, producing hydrogen gas. This is a classic example of a redox reaction where the acid acts as an oxidizing agent. For instance, the reaction between hydrochloric acid and zinc:
2HCl(aq) + Zn(s) → ZnCl₂(aq) + H₂(g) -
Change in pH: Acids have a pH less than 7, with stronger acids having lower pH values. The pH scale is a logarithmic scale that reflects the concentration of H⁺ ions.
-
Reaction with bases: Acids react with bases in neutralization reactions, producing salt and water. This is a fundamental reaction in acid-base chemistry, and it's a key application of the Arrhenius theory.
Limitations of the Arrhenius Theory
Despite its importance and simplicity, the Arrhenius theory has limitations. It only considers aqueous solutions; it cannot explain the acidic behavior of substances that don't contain hydrogen or don't release hydrogen ions in water. For instance, some substances exhibit acidic behavior in non-aqueous solvents. The theory also doesn't adequately explain the behavior of amphoteric substances, which can act as both acids and bases.
Because of these limitations, more comprehensive theories have been developed, such as the Brønsted-Lowry theory and the Lewis theory. These theories provide broader definitions of acids and bases that encompass a wider range of chemical systems.
The Brønsted-Lowry and Lewis Theories: Expanding the Definition
The Brønsted-Lowry theory defines acids as proton donors and bases as proton acceptors. This expands the definition beyond aqueous solutions and includes reactions where protons are transferred between molecules. The Lewis theory, even more general, defines acids as electron-pair acceptors and bases as electron-pair donors. This allows for a much wider range of acid-base reactions, including those that don't involve protons at all.
However, it's crucial to remember that the Arrhenius theory remains a vital stepping stone in understanding acid-base chemistry. Its simplicity provides a solid foundation upon which more advanced theories are built. Learning the Arrhenius definition is essential for grasping the core concepts and for laying the groundwork to understand more complex acid-base reactions and concepts.
Conclusion: The Enduring Legacy of Arrhenius
Svante Arrhenius's theory, despite its limitations, provided a revolutionary framework for understanding acids. The simple definition—an acid is a substance that increases the concentration of hydrogen ions (or more accurately, hydronium ions) when dissolved in water—laid the foundation for countless advancements in chemistry. While more comprehensive theories have been developed, the Arrhenius theory remains a critical element in the education and understanding of acid-base chemistry. Its simplicity and clarity make it an invaluable tool for introducing students to this fundamental concept, highlighting the importance of understanding the basic principles before delving into more nuanced models. The legacy of Arrhenius continues to shape our understanding of the chemical world around us.
Latest Posts
Latest Posts
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about According To Arrhenius Theory What Is An Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
