According To Dalton's Atomic Theory Atoms
Muz Play
Mar 29, 2025 · 6 min read
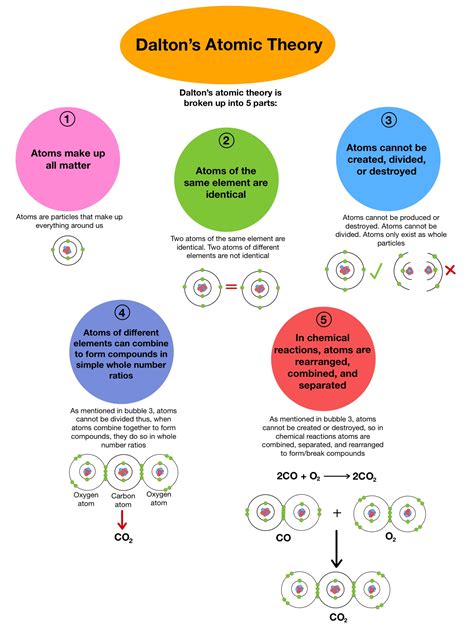
Table of Contents
According to Dalton's Atomic Theory: A Deep Dive into the Foundations of Modern Chemistry
John Dalton's atomic theory, proposed in the early 1800s, revolutionized our understanding of matter. While some aspects have been refined or superseded by later discoveries, its core tenets remain foundational to modern chemistry. This article delves into Dalton's atomic theory, exploring its postulates, their implications, and the scientific advancements that built upon – and in some cases, challenged – his groundbreaking work.
The Five Postulates of Dalton's Atomic Theory
Dalton's theory, published in his seminal work A New System of Chemical Philosophy, rested on five key postulates:
-
All matter is made of atoms: This seemingly simple statement was revolutionary at the time. Before Dalton, the existence of atoms was largely philosophical speculation. Dalton provided a concrete, scientific basis for the atomic concept, tying it directly to observable chemical phenomena. He posited that atoms are indivisible and indestructible particles – a notion that would later require modification.
-
All atoms of a given element are identical in mass and properties: This postulate stated that all atoms of a specific element (e.g., all hydrogen atoms) are exactly alike in their mass and other characteristics. This implies uniformity within an element – a critical concept for understanding chemical reactions. However, the discovery of isotopes later revealed the limitations of this statement. Isotopes are atoms of the same element with different numbers of neutrons, thus varying in mass.
-
Atoms of different elements have different masses and different properties: This postulate highlighted the fundamental difference between elements. Each element possesses unique atomic properties distinguishing it from all others. This explains the unique behaviors of different substances in chemical reactions. This remains a cornerstone of modern chemistry.
-
Atoms combine in simple, whole-number ratios to form chemical compounds: This postulate is central to the law of definite proportions, which states that a given compound always contains the same elements in the same proportion by mass. Dalton's theory elegantly explains this observation by proposing that atoms combine in fixed ratios to form molecules. For example, water always has a 2:1 ratio of hydrogen to oxygen atoms (H₂O).
-
Atoms are neither created nor destroyed in chemical reactions: This postulate reflects the law of conservation of mass, stating that mass is neither gained nor lost during a chemical reaction. The total mass of reactants equals the total mass of products. This principle is crucial for balancing chemical equations and understanding the quantitative aspects of chemical transformations.
Implications and Advancements Based on Dalton's Theory
Dalton's theory had profound implications for the development of chemistry:
-
Quantitative Analysis: It provided a framework for understanding chemical reactions quantitatively. The ability to predict the ratios in which atoms combine allowed for the development of stoichiometry – the study of the quantitative relationships between reactants and products in chemical reactions.
-
Development of the Periodic Table: While not directly responsible for the creation of the periodic table, Dalton's work laid the groundwork. The understanding of atomic weights (masses) and the different properties of elements became essential for organizing the elements into the periodic table.
-
Understanding Chemical Formulas and Equations: Dalton's theory provided the foundation for writing chemical formulas and balancing chemical equations. These are essential tools used daily by chemists.
-
Law of Multiple Proportions: Dalton's theory also explained the law of multiple proportions, which states that when two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in a simple ratio. For example, carbon and oxygen can form carbon monoxide (CO) and carbon dioxide (CO₂).
Limitations and Refinements of Dalton's Theory
Despite its monumental contribution, Dalton's theory had limitations:
-
The Subatomic World: Dalton believed atoms were indivisible and indestructible. Later discoveries revealed the subatomic structure of the atom, with protons, neutrons, and electrons. This refuted the indivisibility aspect of his theory.
-
Isotopes: The discovery of isotopes showed that atoms of the same element could have different masses. This contradicted his postulate that all atoms of a given element are identical.
-
Atomic Structure: Dalton's theory did not address the arrangement or behavior of electrons within the atom. The Bohr model and subsequent quantum mechanical models provided more sophisticated descriptions of atomic structure.
-
Molecular Structures: While Dalton's theory explained the existence of molecules formed from the combination of atoms, it didn't provide a mechanism for understanding the three-dimensional structure of molecules and their influence on chemical properties.
The Legacy of Dalton's Atomic Theory
Despite its limitations, Dalton's atomic theory represents a pivotal moment in the history of science. It transformed chemistry from a largely qualitative science into a quantitative discipline. His postulates, while not completely accurate in the light of later discoveries, provided a robust framework that propelled the field forward. The theory's success in explaining fundamental chemical laws highlighted the power of scientific models in explaining natural phenomena.
Beyond the Postulates: Understanding the Scientific Method
Dalton's work beautifully illustrates the scientific method in action. He didn't just propose a theory; he built it upon existing experimental observations, namely the laws of conservation of mass and definite proportions. His theory offered explanations for these laws, and importantly, it made testable predictions that could either confirm or refute his model. This cyclical process of observation, hypothesis formation, experimentation, and refinement is at the heart of scientific progress.
The Evolution of Atomic Theory: From Dalton to Quantum Mechanics
The journey of atomic theory extends far beyond Dalton's contribution. Subsequent discoveries and theoretical advancements continually refined and expanded our understanding of the atom. Thomson's discovery of the electron, Rutherford's gold foil experiment revealing the nucleus, and Bohr's model incorporating quantized energy levels are just some of the significant milestones. The culmination of these advancements led to the development of quantum mechanics, providing the most comprehensive and accurate description of the atom to date.
Connecting Dalton's Theory to Modern Chemistry
While our understanding of the atom has evolved dramatically since Dalton's time, the core ideas underpinning his theory remain relevant. The concepts of atoms combining in simple whole-number ratios to form molecules, the unique properties of different elements, and the conservation of mass during chemical reactions are all fundamental to modern chemistry.
Modern chemistry builds upon the foundational concepts established by Dalton. While we now understand the complexities of subatomic particles and quantum mechanics, the principles of stoichiometry, chemical formulas, and balanced equations all find their roots in Dalton's pioneering work.
Conclusion: A Lasting Impact
John Dalton's atomic theory represents a pivotal moment in the history of science. While not without flaws, it provided a robust framework for understanding chemical reactions and laid the groundwork for countless advancements in chemistry and related fields. His work exemplifies the power of scientific inquiry, highlighting the importance of observation, hypothesis formation, and rigorous testing in the pursuit of scientific knowledge. Dalton's legacy continues to shape our understanding of the world at the atomic level, even as our knowledge continues to evolve. The fundamental concepts he introduced remain integral to the way chemists think and work today, a testament to the enduring power of his revolutionary ideas.
Latest Posts
Latest Posts
-
Can Sample Variance Be Smaller Than Population Variance
Mar 31, 2025
-
Integrated Rate Law For Zero Order Reaction
Mar 31, 2025
-
Is Melting Point Chemical Or Physical
Mar 31, 2025
-
What Is The Difference Between Density Dependent And Density Independent
Mar 31, 2025
-
10 Common Diseases That Can Cause A Secondary Immunodeficiency
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about According To Dalton's Atomic Theory Atoms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
