Acetals Are Prepared From Ketones And Alcohols.
Muz Play
Mar 16, 2025 · 6 min read
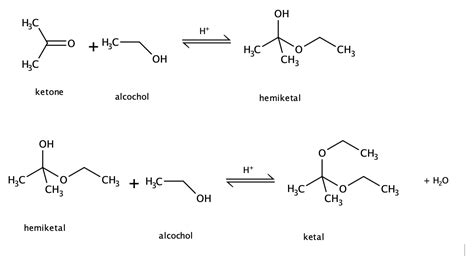
Table of Contents
Acetals: Synthesis from Ketones and Alcohols – A Deep Dive
Acetals are vital functional groups in organic chemistry, boasting a wide range of applications in various fields. Their synthesis, predominantly from ketones and alcohols, is a crucial reaction with significant implications in both academic research and industrial processes. This comprehensive article delves into the intricacies of acetal formation, exploring the mechanisms, reaction conditions, and diverse applications of these valuable compounds.
Understanding Acetals and their Structure
Before diving into the synthesis, let's establish a clear understanding of what acetals are. An acetal is a functional group characterized by a carbon atom bonded to two alkoxy groups (-OR) and two other substituents, which can be alkyl or aryl groups. In simpler terms, it's a central carbon atom surrounded by two ether-like linkages and two additional groups. The formation of an acetal always involves the reaction of a carbonyl compound (aldehyde or ketone) with two equivalents of an alcohol.
Importantly, differentiating between acetals and hemiacetals is crucial. A hemiacetal is an intermediate product formed when a carbonyl compound reacts with only one equivalent of an alcohol. It possesses both an alcohol (-OH) and an ether (-OR) group attached to the same carbon. Further reaction of the hemiacetal with another equivalent of alcohol, often catalyzed by an acid, results in the formation of the acetal.
The Mechanism of Acetal Formation: A Step-by-Step Guide
The formation of acetals from ketones and alcohols is typically an acid-catalyzed process. The mechanism proceeds through several key steps:
Step 1: Protonation of the Carbonyl Group
The reaction begins with the protonation of the carbonyl oxygen of the ketone by a strong acid catalyst, such as sulfuric acid (H₂SO₄) or p-toluenesulfonic acid (TsOH). This protonation increases the electrophilicity of the carbonyl carbon, making it more susceptible to nucleophilic attack.
Step 2: Nucleophilic Attack by the Alcohol
The oxygen atom of the alcohol acts as a nucleophile, attacking the electrophilic carbonyl carbon. This forms a tetrahedral intermediate, where the carbonyl double bond is broken.
Step 3: Proton Transfer and Water Elimination
A proton transfer occurs within the tetrahedral intermediate, followed by the elimination of a water molecule. This step generates a hemiacetal. The hemiacetal is itself usually unstable and readily proceeds to the acetal.
Step 4: Protonation of the Hemiacetal Hydroxyl Group
The hydroxyl group of the hemiacetal is then protonated by the acid catalyst, making it a better leaving group.
Step 5: Nucleophilic Attack by a Second Alcohol Molecule
Another molecule of alcohol acts as a nucleophile, attacking the protonated hydroxyl group. This results in the formation of a new tetrahedral intermediate.
Step 6: Deprotonation and Acetal Formation
Finally, deprotonation by a base (often the conjugate base of the acid catalyst) yields the stable acetal product and regenerates the acid catalyst.
Detailed Illustration: Imagine a ketone, say acetone (CH₃)₂CO, reacting with methanol (CH₃OH). The mechanism would follow the steps above, ultimately yielding 2,2-dimethoxypropane, a common acetal. The key is the addition of two methanol molecules across the carbonyl bond of the acetone, resulting in the replacement of the oxygen atom with two methoxy (-OCH₃) groups.
Reaction Conditions and Optimization: Crucial Factors for Successful Synthesis
The successful synthesis of acetals hinges on careful control of several reaction parameters:
-
Acid Catalyst: The choice of acid catalyst is crucial. Strong acid catalysts like sulfuric acid are effective but can lead to side reactions. Weaker acids like p-toluenesulfonic acid (TsOH) are often preferred for their selectivity. The concentration of the acid also plays a role, with excess acid potentially leading to undesired side products.
-
Alcohol Concentration: The reaction requires at least two equivalents of alcohol to ensure complete conversion to the acetal. An excess of alcohol can often drive the reaction to completion and enhance the yield.
-
Temperature: The reaction temperature influences the rate of acetal formation. While elevated temperatures can accelerate the reaction, they may also lead to unwanted side reactions or decomposition of the reactants or products. Optimization is crucial.
-
Solvent: The choice of solvent can affect the solubility of the reactants and the reaction rate. Common solvents include the alcohol itself or inert solvents like benzene or dichloromethane. Aqueous solvents can also sometimes be employed.
-
Removal of Water: The equilibrium of acetal formation is significantly influenced by the presence of water. Since water is a product of the reaction, its removal (e.g., using a Dean-Stark apparatus for azeotropic distillation) will drive the equilibrium to favor acetal formation, thereby increasing the yield.
Applications of Acetals: A Multifaceted Functional Group
Acetals find widespread use in organic synthesis and beyond. Their remarkable stability under basic conditions, coupled with their ability to be selectively cleaved under acidic conditions, makes them valuable protecting groups for carbonyl compounds.
1. Protecting Groups: In multi-step synthesis, carbonyl groups in a molecule can interfere with other reactions. Acetals effectively mask (protect) these carbonyl groups until they are needed later in the synthesis. The protecting group can then be removed under acidic conditions to regenerate the ketone.
2. Synthesis of Complex Molecules: Acetals are frequently employed as intermediates in the synthesis of complex organic molecules, including pharmaceuticals and natural products. Their selective formation and cleavage offer precise control over reaction pathways.
3. Polymer Chemistry: Acetals are used in the synthesis of various polymers, including polyacetals which find applications in packaging and engineering plastics.
4. Flavor and Fragrance Chemistry: Many acetal compounds contribute to the characteristic scents and flavors of fruits and other natural products. Their synthesis and incorporation into perfumes and food additives is a significant area.
5. Medicinal Chemistry: Acetal functional groups are present in many biologically active molecules, playing crucial roles in their interactions with biological targets. This makes them a subject of ongoing research in drug discovery and development.
6. Carbohydrate Chemistry: Many carbohydrates contain acetal linkages, and their study is crucial for understanding the structure and reactivity of these essential biomolecules.
Specific Examples of Acetal Synthesis and Applications
Let's consider a few concrete examples to illustrate the versatility of acetal formation:
-
Protection of a ketone in a synthesis: If a molecule contains both a ketone and an alcohol, the ketone might need protection before the alcohol can undergo reaction. Forming an acetal from the ketone effectively shields it from unwanted reactivity.
-
The synthesis of a specific pharmaceutical compound: The precise introduction of an acetal group into a drug molecule might be required to enhance its bioavailability, target specificity, or other desirable properties.
-
The preparation of a polymer with enhanced thermal stability: The incorporation of acetal linkages into a polymer backbone can contribute to improved thermal stability and resistance to degradation.
Conclusion: The Importance of Acetals in Organic Chemistry
The synthesis of acetals from ketones and alcohols is a fundamental and highly versatile reaction in organic chemistry. The profound importance of acetals lies in their stability under basic conditions, their selective cleavage under acidic conditions, and their wide array of applications across diverse fields. From protecting sensitive functional groups in multistep synthesis to playing crucial roles in the preparation of pharmaceuticals and polymers, acetals stand as a testament to the power and elegance of organic chemistry. Mastering the intricacies of acetal formation and manipulation provides synthetic chemists with a powerful tool for building complex molecules and exploring the fascinating realm of organic chemistry. Continued research in this area promises to yield even more exciting discoveries and applications in the future.
Latest Posts
Latest Posts
-
Signal Transduction Takes Place In The
Mar 16, 2025
-
Which One Increases Number Of Particles Avilabile To React
Mar 16, 2025
-
Factors That Affect Growth Of Microorganisms
Mar 16, 2025
-
High Quality Definition Color Phase Plots Complex Analysis
Mar 16, 2025
-
How To Make A Catalytic Triad
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Acetals Are Prepared From Ketones And Alcohols. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
