Which One Increases Number Of Particles Avilabile To React
Muz Play
Mar 16, 2025 · 5 min read
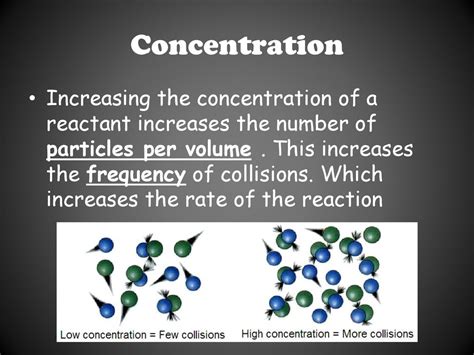
Table of Contents
Which Factor Increases the Number of Particles Available to React?
Understanding reaction rates is crucial in chemistry and many related fields. A fundamental principle dictates that the speed of a chemical reaction is directly influenced by the number of particles available to collide and react. This article delves deep into the factors that significantly increase the number of particles available for reaction, focusing on concentration, surface area, and temperature, with a nuanced look at their interconnectedness and implications.
The Importance of Particle Collisions
Chemical reactions, at their core, involve the collision and interaction of reactant particles (atoms, molecules, or ions). The more frequently these particles collide with sufficient energy, the faster the reaction proceeds. Therefore, any factor that increases the frequency and effectiveness of these collisions will inherently increase the reaction rate. The number of particles readily available for collision is the primary driver of this frequency.
Concentration: More Particles, More Reactions
Concentration is arguably the most straightforward factor influencing the number of particles available for reaction. Concentration refers to the amount of solute (the substance being dissolved) present in a given volume of solvent (the substance doing the dissolving) or solution. A higher concentration means more reactant particles are packed into a specific volume.
How Concentration Affects Collision Frequency
Imagine two scenarios: a dilute solution (low concentration) and a concentrated solution (high concentration). In the dilute solution, reactant particles are sparsely distributed, leading to infrequent collisions. In the concentrated solution, particles are densely packed, resulting in significantly more frequent collisions. This increased collision frequency directly translates to a higher reaction rate.
Practical Examples of Concentration Effects
The impact of concentration is vividly demonstrated in various everyday phenomena:
- Burning: A wood fire burns faster in pure oxygen (high concentration of oxygen) than in air (lower concentration of oxygen).
- Rusting: Iron rusts faster in humid environments (higher concentration of water vapor) than in dry ones.
- Cooking: Food cooks faster in a pressure cooker (higher concentration of water molecules due to increased pressure) compared to open-air cooking.
Mathematical Representation: Rate Laws
The relationship between concentration and reaction rate is often mathematically expressed through rate laws. For a simple reaction A + B → C, a rate law might look like:
Rate = k[A][B]
where:
Rateis the speed of the reaction.kis the rate constant (dependent on temperature and other factors).[A]and[B]represent the concentrations of reactants A and B.
This equation illustrates that the reaction rate is directly proportional to the concentrations of both reactants. Doubling the concentration of A or B would roughly double the reaction rate.
Surface Area: Expanding Accessibility
For reactions involving solids, surface area plays a critical role in determining the number of particles accessible for reaction. A larger surface area means more reactant particles are exposed and available for collision with other reactants.
The Impact of Particle Size
Consider a solid reactant like a metal. A single, large piece of metal has a relatively small surface area compared to the same mass of metal finely powdered. The powder exposes a significantly larger surface area, increasing the number of metal particles available to react with other substances.
Examples of Surface Area's Influence
The importance of surface area is evident in:
- Combustion: Finely divided wood shavings ignite and burn much more readily than a large log because of their larger surface area.
- Dissolution: A sugar cube dissolves slower than granulated sugar because the granulated sugar has a vastly larger surface area.
- Catalysis: Heterogeneous catalysts (catalysts in a different phase than the reactants) typically function by providing a large surface area for reactant molecules to adsorb onto, facilitating the reaction.
Temperature: Boosting Kinetic Energy
Temperature directly impacts the number of particles available to react, not by changing their quantity, but by influencing their kinetic energy. Higher temperatures translate to faster-moving particles with more kinetic energy.
The Role of Activation Energy
Every chemical reaction has an activation energy (Ea), which is the minimum energy required for reactant particles to successfully collide and form products. Many collisions occur without resulting in a reaction because the colliding particles lack sufficient energy to overcome the activation energy barrier.
Temperature and Collision Effectiveness
Increasing the temperature increases the average kinetic energy of the particles. This means:
- More frequent collisions: Particles move faster, increasing the frequency of collisions.
- More effective collisions: A larger proportion of collisions possess sufficient energy to surpass the activation energy barrier, resulting in a higher percentage of successful reactions.
The Arrhenius Equation
The quantitative relationship between temperature and reaction rate is described by the Arrhenius equation:
k = A * e^(-Ea/RT)
Where:
kis the rate constant.Ais the pre-exponential factor (related to collision frequency).Eais the activation energy.Ris the gas constant.Tis the absolute temperature (in Kelvin).
This equation demonstrates the exponential dependence of the rate constant (and hence the reaction rate) on temperature. A small increase in temperature can lead to a substantial increase in reaction rate.
Interconnectedness of Factors
It's crucial to understand that these factors – concentration, surface area, and temperature – are not independent. They often influence each other and collectively affect the number of particles available for reaction.
For example, increasing the temperature of a concentrated solution will not only increase the kinetic energy of the particles but also potentially increase the rate of diffusion, leading to even more frequent and effective collisions. Similarly, increasing the surface area of a solid reactant at higher temperatures can further accelerate the reaction rate.
Conclusion: Optimizing Reaction Rates
The number of particles available for reaction is a fundamental determinant of reaction rates. By manipulating concentration, surface area, and temperature, we can effectively control and optimize chemical reaction speeds. Understanding these factors and their interplay is vital in various fields, from industrial chemical processes to biological systems. Careful consideration of these factors allows for efficient and controlled chemical transformations, crucial for numerous applications. Further exploration into specific reaction mechanisms and the intricacies of intermolecular forces would provide even deeper insights into this fundamental aspect of chemistry.
Latest Posts
Latest Posts
-
According To The Kinetic Theory Of Gases
Mar 17, 2025
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
-
For An Exothermic Reaction The Products
Mar 17, 2025
-
Fallacies Divide Into Roughly Two Kinds
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which One Increases Number Of Particles Avilabile To React . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
