Acetic Acid And Sodium Acetate Buffer
Muz Play
Mar 26, 2025 · 6 min read
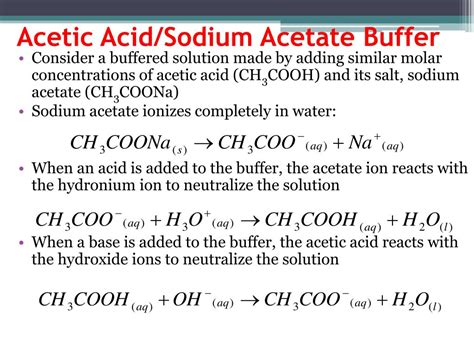
Table of Contents
Acetic Acid and Sodium Acetate Buffer: A Deep Dive
The acetic acid/sodium acetate buffer system is a classic example of a weak acid-conjugate base buffer solution. Understanding its properties and applications is crucial for anyone studying chemistry, biochemistry, or related fields. This comprehensive guide will explore the intricacies of this buffer system, delving into its composition, preparation, buffering capacity, applications, and limitations.
Understanding Buffer Solutions
Before diving into the specifics of the acetic acid/sodium acetate buffer, let's establish a foundational understanding of buffer solutions. A buffer solution is an aqueous solution that resists changes in pH upon the addition of small amounts of acid or base. This resistance to pH change is critical in many biological and chemical processes where maintaining a stable pH is essential. Buffers achieve this through the presence of a weak acid and its conjugate base (or a weak base and its conjugate acid).
The key to a buffer's effectiveness lies in the equilibrium between the weak acid and its conjugate base. When a strong acid is added, the conjugate base reacts to neutralize it, minimizing the pH change. Conversely, when a strong base is added, the weak acid reacts to neutralize it, again minimizing the pH change.
The Acetic Acid/Sodium Acetate Buffer System: A Detailed Look
The acetic acid/sodium acetate buffer is a common example employing the weak acid, acetic acid (CH₃COOH), and its conjugate base, the acetate ion (CH₃COO⁻), usually provided by the salt sodium acetate (CH₃COONa).
Composition and Preparation
Preparing an acetic acid/sodium acetate buffer involves mixing appropriate amounts of acetic acid and sodium acetate. The exact ratio depends on the desired pH of the buffer. The pH of the buffer can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([A⁻]/[HA])
Where:
- pH is the desired pH of the buffer
- pKa is the negative logarithm of the acid dissociation constant (Ka) of acetic acid (approximately 4.76 at 25°C)
- [A⁻] is the concentration of the conjugate base (acetate ion)
- [HA] is the concentration of the weak acid (acetic acid)
By manipulating the ratio of [A⁻] to [HA], we can fine-tune the buffer's pH. For example, a buffer with equal concentrations of acetic acid and sodium acetate will have a pH close to the pKa (4.76).
A typical preparation might involve dissolving specific amounts of glacial acetic acid and sodium acetate trihydrate in distilled water, ensuring the final volume and concentrations are accurately measured.
Buffering Capacity
The buffering capacity refers to the amount of acid or base a buffer can neutralize before experiencing a significant pH change. The buffering capacity is highest when the concentrations of the weak acid and its conjugate base are equal ([A⁻] = [HA]), which occurs when the pH is equal to the pKa. As the ratio deviates significantly from 1:1, the buffering capacity diminishes.
Several factors influence the buffering capacity, including:
- Concentrations of the acid and conjugate base: Higher concentrations lead to higher buffering capacity.
- The ratio of acid to conjugate base: A ratio closer to 1:1 provides the highest capacity.
- The strength of the weak acid: A weaker acid will generally have a lower buffering capacity.
Applications of the Acetic Acid/Sodium Acetate Buffer
The acetic acid/sodium acetate buffer finds widespread applications across various scientific disciplines due to its relatively simple preparation, readily available components, and suitable pH range:
-
Biochemical Experiments: Maintaining a stable pH is crucial in many biochemical reactions and processes. This buffer is commonly used in enzyme assays, protein purifications, and cell cultures. Its pH range makes it suitable for many biological systems.
-
Analytical Chemistry: The buffer is often used in titrations and other analytical techniques that require a stable pH environment. Its predictable behavior makes it a reliable choice for quantitative analysis.
-
Food Preservation: The acidic nature of acetic acid contributes to food preservation, inhibiting the growth of microorganisms. While not solely used as a buffer in this context, the combination of acetic acid and sodium acetate can contribute to a stable pH environment in certain food products.
-
Textile Industry: Acetic acid and acetate salts are used in various textile dyeing and finishing processes. The buffer system might be employed to control the pH during these processes.
-
Photography: The buffer can play a role in certain photographic development processes where pH control is vital.
Limitations of the Acetic Acid/Sodium Acetate Buffer
While versatile, the acetic acid/sodium acetate buffer does have limitations:
-
Limited pH range: The buffer's effectiveness is largely confined to a pH range approximately one pH unit above and below its pKa (3.76 - 5.76). Outside this range, its buffering capacity significantly decreases.
-
Temperature dependence: The pKa of acetic acid, and thus the pH of the buffer, varies with temperature. Precise pH control might require temperature compensation.
-
Ionic strength: The presence of high concentrations of other ions can affect the buffer's performance. This needs consideration when designing experimental setups.
-
Sensitivity to contamination: Introducing strong acids or bases beyond the buffer's capacity will cause significant pH shifts. Careful handling and cleanliness are crucial.
Comparing Acetic Acid/Sodium Acetate Buffer with Other Buffer Systems
Several other buffer systems exist, each with its strengths and weaknesses. The choice of buffer depends entirely on the specific application and the required pH range. Here’s a brief comparison:
-
Phosphate buffers: These buffers are commonly used in biological systems due to their biocompatibility and wide pH range (pH 5.8 - 8.0). However, they can inhibit enzymatic activity in some cases.
-
Tris buffers: Tris (tris(hydroxymethyl)aminomethane) buffers are frequently employed in biochemical research due to their relatively good buffering capacity and biocompatibility. However, their pKa is temperature-dependent, necessitating adjustments.
-
Citrate buffers: These buffers are useful in a lower pH range (pH 2.2 - 6.2) and are often used in food and beverage applications.
-
Carbonate buffers: These buffers operate effectively within a pH range (pH 9.2 - 10.8) and are used in applications requiring a higher pH.
The selection of the optimal buffer system is crucial. While acetic acid/sodium acetate offers simplicity and suitability for a specific pH range, other buffers might be more appropriate based on the specific application requirements.
Advanced Considerations: Factors Affecting Buffer Performance
Beyond the basics, several factors can subtly influence the performance of the acetic acid/sodium acetate buffer:
-
Ionic Strength: The ionic strength of the buffer solution affects the activity coefficients of the ions, influencing the effective concentrations and ultimately the buffer's pH. High ionic strength can alter the buffer’s performance.
-
Temperature Effects: The pKa of acetic acid is temperature-dependent. Changes in temperature can shift the equilibrium and affect the buffer's pH. Careful temperature control is crucial for precise pH maintenance.
-
Solvent Effects: Using non-aqueous solvents can significantly alter the acid dissociation constant of acetic acid and the buffer's behaviour.
-
Presence of other components: The introduction of other ions or molecules can interact with the buffer components, affecting the equilibrium and the overall pH. Careful consideration of potential interactions is essential.
Conclusion
The acetic acid/sodium acetate buffer system is a cornerstone of chemistry and biochemistry, offering a simple yet effective means of controlling pH in various applications. Understanding its composition, preparation, buffering capacity, and limitations is paramount for successful implementation. While this system excels within its optimal pH range, researchers and practitioners must remember to consider the various factors that can influence its performance and choose the most appropriate buffer system for each specific application. Always prioritize accurate measurements, controlled conditions, and a thorough understanding of the underlying principles to ensure the reliable functioning of the buffer system in your experiments and applications.
Latest Posts
Latest Posts
-
How To Determine A Strong Acid
Mar 29, 2025
-
Punnett Squares Crosses Involving One Trait
Mar 29, 2025
-
When Pressure Increases Then The Volume Must
Mar 29, 2025
-
How To Check For Linear Independence
Mar 29, 2025
-
Find The Characteristic Polynomial Of The Matrix
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Acetic Acid And Sodium Acetate Buffer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
