Acid Catalyzed Hydration Of Alkynes Mechanism
Muz Play
Mar 23, 2025 · 5 min read
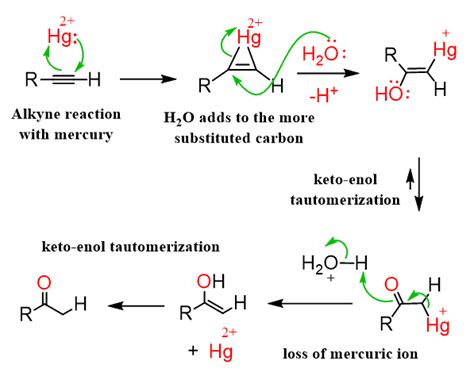
Table of Contents
Acid-Catalyzed Hydration of Alkynes: A Comprehensive Guide
The acid-catalyzed hydration of alkynes is a fundamental organic chemistry reaction that transforms alkynes into ketones or aldehydes. This process is a crucial synthetic tool, offering a pathway to synthesize valuable carbonyl compounds from readily available alkyne starting materials. This detailed guide will delve into the mechanism, regioselectivity, stereochemistry, and practical applications of this reaction. We'll also explore variations and limitations to provide a complete understanding.
Understanding the Reaction: Alkynes to Carbonyls
The acid-catalyzed hydration of alkynes involves the addition of water across the carbon-carbon triple bond. This addition is facilitated by a strong acid catalyst, typically a mineral acid like sulfuric acid (H₂SO₄) or a combination of sulfuric acid and mercury(II) sulfate (HgSO₄). The reaction generally proceeds via a Markovnikov addition, meaning that the hydroxyl group (–OH) adds to the more substituted carbon atom of the alkyne. However, as we'll see, this regioselectivity can be influenced by certain factors.
The overall reaction can be represented as follows:
RC≡CR' + H₂O --(H⁺ catalyst)--> R-CO-CH₂R' or RCH₂-CO-R'
This equation shows the general transformation; the specific product (ketone or aldehyde) depends on the substitution pattern of the starting alkyne. A terminal alkyne (RC≡CH) will produce a methyl ketone, while an internal alkyne (RC≡CR') will yield a ketone.
The Mechanism: A Step-by-Step Analysis
The acid-catalyzed hydration of alkynes proceeds through a series of well-defined steps:
Step 1: Protonation of the Alkyne
The reaction initiates with the protonation of the alkyne's triple bond by the strong acid catalyst (H⁺). This step generates a vinyl cation, a carbocation where the positive charge resides on a carbon atom that is sp hybridized. The more substituted vinyl cation is formed preferentially due to its greater stability (hyperconjugation). This step determines the regioselectivity of the reaction.
RC≡CR' + H⁺ <--> RC⁺=CHR'
Step 2: Nucleophilic Attack by Water
Next, a water molecule acts as a nucleophile, attacking the electrophilic carbon atom of the vinyl cation. This step forms a protonated enol. The oxygen atom of the water molecule donates its lone pair of electrons to form a new bond with the carbocation.
RC⁺=CHR' + H₂O <--> RC(OH⁺)=CHR'
Step 3: Deprotonation
In the final step, a base (water or the conjugate base of the acid catalyst) abstracts a proton from the protonated enol. This results in the formation of an enol, which is a molecule containing a hydroxyl group and a double bond.
RC(OH⁺)=CHR' + H₂O <--> RC(OH)=CHR' + H₃O⁺
Step 4: Keto-Enol Tautomerization
The enol produced in the previous step is unstable and quickly undergoes tautomerization to form a more stable keto form. This tautomerization involves the transfer of a proton from the hydroxyl group to the alpha-carbon, accompanied by the shift of a double bond. This is a rapid equilibrium that favors the keto form.
RC(OH)=CHR' <--> R-CO-CH₂R'
The final product is either a ketone or an aldehyde, depending on the structure of the starting alkyne. This mechanism beautifully illustrates the interplay of electrophilic addition, nucleophilic attack, and tautomerization in organic chemistry.
Regioselectivity: Markovnikov's Rule in Action
As mentioned earlier, the acid-catalyzed hydration of alkynes generally follows Markovnikov's rule. This means that the hydroxyl group (–OH) adds to the more substituted carbon atom of the alkyne. This regioselectivity is dictated by the stability of the vinyl cation intermediate formed in Step 1. The more substituted vinyl cation is more stable due to hyperconjugation and inductive effects, leading to its preferential formation and subsequently, the Markovnikov product.
For example, the hydration of 2-butyne will predominantly yield 2-butanone (a ketone):
CH₃C≡CCH₃ + H₂O --(H⁺ catalyst)--> CH₃COCH₂CH₃
However, it's important to note that in the presence of certain catalysts or under specific conditions, the reaction might deviate from Markovnikov's rule. This will be discussed further in the following section.
Stereochemistry: The Significance of Enol Formation
The acid-catalyzed hydration of alkynes generally produces ketones or aldehydes with no stereochemical implications, in contrast to alkene hydration. This is due to the keto-enol tautomerization step, which effectively destroys any stereochemical information present in the enol intermediate. The subsequent formation of the ketone or aldehyde lacks any stereocenters.
Variations and Limitations
While the acid-catalyzed hydration of alkynes is a powerful method, it does have certain limitations and variations:
Mercury(II) Sulfate (HgSO₄) as a Catalyst
Often, mercury(II) sulfate (HgSO₄) is used in conjunction with sulfuric acid (H₂SO₄). This combination enhances the reaction rate and improves the yield, particularly for less reactive alkynes. The mercury ion interacts with the alkyne, facilitating the formation of the vinyl cation and promoting the addition of water.
Limitations: Steric Hindrance and Reactivity
Sterically hindered alkynes might react more slowly or with lower yields due to the difficulty of the nucleophile approaching the bulky vinyl cation intermediate. Similarly, some alkynes might be less reactive towards hydration, requiring more forcing conditions or alternative catalytic systems.
Regioselectivity Deviation: Anti-Markovnikov Addition
Although less common, anti-Markovnikov addition can be achieved under specific conditions. Using certain catalysts or employing specific reaction conditions might influence the regioselectivity and produce the less stable vinyl cation leading to the anti-Markovnikov product. This is a more specialized area and often requires tailored catalytic systems.
Applications and Synthetic Importance
The acid-catalyzed hydration of alkynes is a valuable tool in organic synthesis, used extensively in the preparation of:
-
Ketones and Aldehydes: As discussed extensively, this is the primary application, allowing access to a broad range of carbonyl compounds from readily available alkynes.
-
Pharmaceutical and Agrochemical Synthesis: Many pharmaceuticals and agrochemicals contain ketone or aldehyde functional groups, making this reaction crucial in their synthesis.
-
Polymer Chemistry: Some polymers are synthesized using monomers prepared through alkyne hydration.
-
Fine Chemical Synthesis: This reaction finds application in the synthesis of various fine chemicals and specialty products.
Conclusion: A Powerful Synthetic Tool
The acid-catalyzed hydration of alkynes is a versatile and important reaction in organic chemistry, providing a direct route to ketones and aldehydes from alkynes. The understanding of its mechanism, regioselectivity, and limitations is crucial for its successful application in various synthetic endeavors. By mastering this reaction and its nuances, chemists can efficiently access a wide range of important carbonyl compounds for use in diverse fields. Further research and development in catalytic systems continue to enhance its scope and applicability, making it an enduringly valuable synthetic tool.
Latest Posts
Latest Posts
-
General Chemistry Principles And Modern Applications By Petrucci
Mar 25, 2025
-
Environmental Factors That Influence Microbial Growth
Mar 25, 2025
-
Electrons In An Atoms Outermost Energy Shells Are Called
Mar 25, 2025
-
Which Kingdoms Contain Organisms That Are Prokaryotes
Mar 25, 2025
-
What Element Is Found In Proteins
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Acid Catalyzed Hydration Of Alkynes Mechanism . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
