Alpha Configuration Bonded To Beta Configuration
Muz Play
Mar 28, 2025 · 7 min read
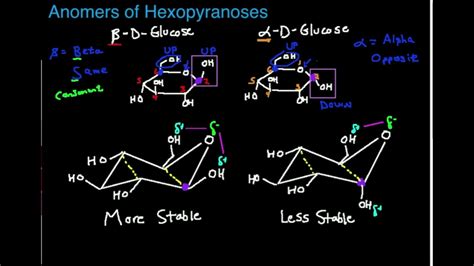
Table of Contents
Alpha Configuration Bonded to Beta Configuration: A Deep Dive into Conformational Isomerism
Conformational isomerism, a fascinating aspect of organic chemistry, deals with molecules possessing the same structural formula but differing in their spatial arrangement due to rotation around single bonds. This article delves into a specific and crucial type of conformational isomerism: the bonding of alpha (α) and beta (β) configurations. While the terms "alpha" and "beta" can refer to a variety of contexts in chemistry, in this discussion, we'll focus on their application within the framework of conformational analysis, particularly as related to cyclic structures and their substituents. Understanding the interplay between α and β configurations is crucial for comprehending molecular properties, reactivity, and biological function.
Understanding Alpha (α) and Beta (β) Configurations
Before exploring the bonding of α and β configurations, let's clearly define what these terms signify within the context of conformational analysis. The designation of α and β typically arises when discussing the relative position of a substituent on a molecule, often a cyclic structure like a sugar or a steroid.
-
Alpha (α): Generally refers to a substituent that is positioned on the same side of a reference plane as another specific group or atom. This is often visualized as being "below" or "closer" in a Fischer projection. The exact meaning depends on the context, and sometimes a specific atom or functional group is designated as the reference point.
-
Beta (β): Conversely, indicates a substituent located on the opposite side of the reference plane compared to the reference group. This is often visualized as being "above" or "further" in a Fischer projection. Again, the context is crucial for precise interpretation.
The Significance of Conformation in α and β Bonding
The interaction between α and β configurations profoundly affects the overall conformation of a molecule. This influence stems from various factors, including steric hindrance, dipole-dipole interactions, hydrogen bonding, and other non-covalent forces. These interactions dictate the molecule's preferred conformation, which, in turn, greatly impacts its chemical reactivity and biological activity.
Steric Hindrance and Conformational Preference
One of the most dominant factors driving conformational preference is steric hindrance. When bulky α and β substituents are close in proximity, they experience steric repulsion, forcing the molecule to adopt a conformation that minimizes this unfavorable interaction. This often involves the substituents adopting positions that are as far apart as sterically possible, leading to specific conformational preferences. Consider, for instance, a cyclohexane ring with α and β substituents. The molecule will preferentially adopt a conformation that places the bulky substituents in equatorial positions to minimize 1,3-diaxial interactions.
Dipole-Dipole Interactions and Hydrogen Bonding
In addition to steric factors, dipole-dipole interactions and hydrogen bonding play a significant role in dictating the preferred conformation. If the α and β substituents possess permanent dipoles, the interaction between these dipoles influences the overall conformation. Molecules will tend to adopt conformations that minimize unfavorable dipole-dipole repulsions and maximize favorable attractions. Similarly, the presence of hydrogen bond donors and acceptors among the α and β substituents can lead to specific conformational preferences driven by the desire to maximize hydrogen bonding interactions.
Examples of α and β Bonding in Different Molecular Systems
The concepts of α and β configurations and their bonding are relevant across a wide range of molecular systems. Let's examine some key examples to illustrate their significance.
1. Carbohydrates: Pyranose and Furanose Rings
Carbohydrates, fundamental biomolecules, often exist in cyclic forms – pyranose (six-membered ring) and furanose (five-membered ring) – where the designation of α and β becomes crucial. The anomeric carbon (the carbon derived from the carbonyl group) plays a key role in determining the anomeric configuration. An α-anomer has the hydroxyl group on the anomeric carbon pointing downwards (trans to the CH<sub>2</sub>OH group), while a β-anomer has it pointing upwards (cis to the CH<sub>2</sub>OH group). The different anomeric configurations lead to distinct physical and chemical properties, impacting the reactivity and biological function of carbohydrates. The bond formed between an α- or β-anomer with another molecule significantly influences the properties of the resulting glycoside.
2. Steroids: A/B Ring Junctions
Steroids, a class of lipids with diverse biological functions, contain multiple fused rings (A, B, C, and D). The relative orientation of the rings (cis or trans) at the A/B ring junction is described using the α and β nomenclature. The A/B ring junction can be cis (α) or trans (β), greatly influencing the overall three-dimensional structure of the steroid molecule. This conformational difference is crucial for the biological activity of steroids, as specific receptor interactions often depend on the precise spatial arrangement of the rings. The configuration of the A/B ring junction has a significant effect on the shape and rigidity of the molecule impacting its ability to bind to its target protein.
3. Amino Acids: Peptide Bond Conformation
While not directly described as α and β configurations in the same way as in cyclic structures, the orientation around the peptide bond in amino acids has a conformational impact that mirrors the principles discussed. The peptide bond exhibits partial double-bond character due to resonance, limiting rotation around this bond. However, the rotation around the bonds adjacent to the peptide bond (φ and ψ angles) determines the conformation of the peptide backbone. Specific conformations, such as α-helices and β-sheets, result from characteristic φ and ψ angles, and the interplay of different amino acid side chains influence the stabilization of these secondary structures. Although not explicitly α and β, the underlying principle of conformational isomerism with significant steric and energetic effects is analogous.
Analytical Techniques for Studying α and β Configurations
Several powerful analytical techniques provide insights into the α and β configurations and their bonding interactions:
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR is an invaluable technique for determining the three-dimensional structure of molecules, including the relative orientations of α and β substituents. The chemical shifts and coupling constants obtained from NMR spectra provide crucial information about the conformation and configuration of the molecule.
-
X-ray Crystallography: X-ray crystallography allows for the determination of the precise three-dimensional structure of a molecule in its crystalline state. This technique provides highly accurate information about the relative positions of α and β substituents and the overall conformation of the molecule.
-
Computational Chemistry: Computational methods, such as molecular mechanics and density functional theory (DFT) calculations, can be used to predict the preferred conformations of molecules with α and β substituents. These calculations provide insights into the energetics of different conformations and help elucidate the factors governing conformational preference.
Biological Implications of α and β Bonding
The α and β configurations and their interactions are of paramount importance in biology. The specific spatial arrangements of molecules, dictated by their α and β configurations, often determine their ability to interact with specific receptors, enzymes, and other biomolecules. Many biological processes rely on the precise recognition of molecules with specific α and β configurations.
Examples include:
-
Enzyme-Substrate Interactions: Enzymes often exhibit high specificity for substrates with particular α and β configurations. The precise fit between the enzyme's active site and the substrate's conformation is essential for efficient catalysis. Incorrect configurations can lead to reduced catalytic efficiency or complete inactivity.
-
Drug Design and Development: Drug design often involves tailoring the α and β configurations of drug molecules to optimize their interactions with target receptors. The specific conformation of a drug molecule is crucial for its efficacy and selectivity.
-
Carbohydrate Recognition: Carbohydrates play critical roles in cellular recognition and signaling processes. The α and β configurations of sugars are crucial determinants of their binding affinities and interactions with carbohydrate-binding proteins (lectins).
Conclusion
The bonding of α and β configurations is a crucial aspect of conformational isomerism with profound implications for the properties, reactivity, and biological function of molecules. Understanding the interplay between these configurations, considering factors such as steric hindrance, dipole-dipole interactions, and hydrogen bonding, is essential for comprehending the behavior of molecules in various contexts. Advanced analytical techniques and computational methods provide powerful tools for investigating α and β configurations and their significance in chemistry and biology. Continued research in this area promises to provide even deeper insights into the complex world of conformational isomerism and its crucial role in various scientific disciplines.
Latest Posts
Latest Posts
-
What Is Reversible And Irreversible Process In Thermodynamics
Mar 31, 2025
-
Moment Of Inertia Of Uniform Rod
Mar 31, 2025
-
Keratin And Collagen Are Examples Of Which Class Of Proteins
Mar 31, 2025
-
Job Order Costing Vs Process Costing
Mar 31, 2025
-
What Is The Chemical Equation For Aerobic Respiration
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Alpha Configuration Bonded To Beta Configuration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
