Amino Acids Are The Monomeric Units Of Which Macromolecules
Muz Play
Mar 18, 2025 · 7 min read
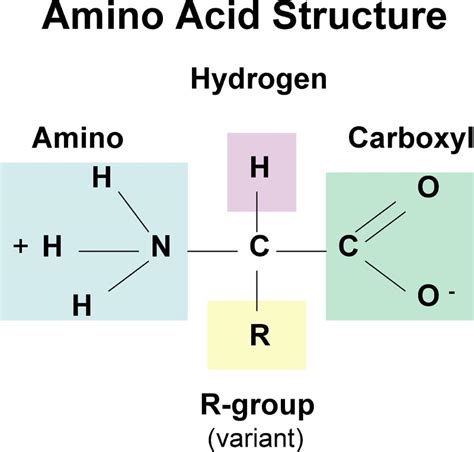
Table of Contents
Amino Acids: The Building Blocks of Proteins – A Deep Dive
Amino acids are the fundamental monomers that make up proteins, one of the four major classes of biological macromolecules. Understanding amino acids is crucial to comprehending the structure, function, and importance of proteins in all living organisms. This comprehensive article delves into the world of amino acids, exploring their structure, properties, functions, and their vital role in building the complex protein molecules that underpin life itself.
The Structure of Amino Acids: A Common Core with Unique Side Chains
All amino acids share a common core structure, consisting of:
- A central carbon atom (α-carbon): This carbon atom is chiral (except for glycine), meaning it has four different groups attached to it.
- An amino group (-NH₂): This group is basic and readily accepts a proton (H⁺).
- A carboxyl group (-COOH): This group is acidic and readily donates a proton (H⁺).
- A hydrogen atom (-H): A simple hydrogen atom bonded to the central carbon.
- A side chain (R-group): This is the variable part of the amino acid, giving each amino acid its unique chemical properties. The R-group can be anything from a simple hydrogen atom (in glycine) to a complex ring structure.
The diversity of the R-group is what determines the unique characteristics of each amino acid. These characteristics influence how the amino acid interacts with other amino acids and its environment, ultimately shaping the protein's overall structure and function.
The 20 Standard Amino Acids: A Diverse Family
There are 20 standard amino acids that are genetically encoded and commonly found in proteins. These amino acids are categorized based on the properties of their side chains:
1. Nonpolar, aliphatic amino acids: These amino acids have hydrophobic (water-fearing) side chains that are typically composed of hydrocarbon chains. Examples include:
- Glycine (Gly, G): The simplest amino acid, with a hydrogen atom as its R-group.
- Alanine (Ala, A): A methyl group (-CH₃) as its R-group.
- Valine (Val, V): A branched isopropyl group as its R-group.
- Leucine (Leu, L): An isobutyl group as its R-group.
- Isoleucine (Ile, I): A branched sec-butyl group as its R-group.
- Methionine (Met, M): Contains a thioether group (-SCH₃) in its side chain.
2. Aromatic amino acids: These amino acids possess aromatic rings in their side chains, making them relatively hydrophobic. Examples include:
- Phenylalanine (Phe, F): Contains a benzene ring.
- Tyrosine (Tyr, Y): Contains a benzene ring with a hydroxyl group (-OH).
- Tryptophan (Trp, W): Contains an indole ring.
3. Polar, uncharged amino acids: These amino acids have side chains that are polar but not charged at physiological pH. They can form hydrogen bonds. Examples include:
- Serine (Ser, S): Contains a hydroxyl group (-OH).
- Threonine (Thr, T): Contains a hydroxyl group (-OH) on a branched carbon.
- Cysteine (Cys, C): Contains a thiol group (-SH), which can form disulfide bonds.
- Asparagine (Asn, N): Contains a carboxamide group (-CONH₂).
- Glutamine (Gln, Q): Contains a carboxamide group (-CONH₂).
4. Positively charged (basic) amino acids: These amino acids have positively charged side chains at physiological pH. Examples include:
- Lysine (Lys, K): Contains a primary amino group (-NH₃⁺) at the end of its side chain.
- Arginine (Arg, R): Contains a guanidinium group, a highly positively charged functional group.
- Histidine (His, H): Contains an imidazole ring, which can be positively or neutrally charged depending on pH.
5. Negatively charged (acidic) amino acids: These amino acids have negatively charged side chains at physiological pH. Examples include:
- Aspartic acid (Asp, D): Contains a carboxyl group (-COO⁻).
- Glutamic acid (Glu, E): Contains a carboxyl group (-COO⁻).
Peptide Bonds: Linking Amino Acids to Form Proteins
Amino acids are linked together by peptide bonds to form polypeptide chains. A peptide bond is a covalent bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH₂) of another amino acid. This reaction releases a molecule of water (H₂O), a process called dehydration synthesis or condensation.
The sequence of amino acids in a polypeptide chain is determined by the genetic code, and this sequence dictates the protein's three-dimensional structure and function. A protein can consist of one or more polypeptide chains, and the arrangement of these chains determines the overall protein structure.
Protein Structure: From Primary to Quaternary
The structure of a protein is crucial for its function, and it's hierarchical, encompassing four levels:
1. Primary Structure: This refers to the linear sequence of amino acids in a polypeptide chain. This sequence is dictated by the genetic code and is fundamental to the higher-order structures. Any change in this sequence (e.g., a single amino acid substitution) can dramatically affect the protein's function.
2. Secondary Structure: This refers to local folding patterns within the polypeptide chain, stabilized by hydrogen bonds between the backbone amide (-NH) and carbonyl (-CO) groups. Common secondary structures include:
- α-helices: Right-handed coiled structures.
- β-sheets: Extended, pleated structures.
- Loops and turns: Irregular regions connecting α-helices and β-sheets.
3. Tertiary Structure: This refers to the overall three-dimensional arrangement of a single polypeptide chain. It's stabilized by various interactions between the side chains (R-groups) of amino acids, including:
- Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from water.
- Hydrogen bonds: Interactions between polar side chains.
- Ionic bonds (salt bridges): Interactions between oppositely charged side chains.
- Disulfide bonds: Covalent bonds between cysteine residues.
4. Quaternary Structure: This refers to the arrangement of multiple polypeptide chains (subunits) in a protein complex. Not all proteins have quaternary structure; some exist as single polypeptide chains. The interactions between subunits are similar to those stabilizing tertiary structure.
The Diverse Functions of Proteins: Essential Roles in Life
Proteins are incredibly versatile molecules, performing a vast array of functions essential for life. Some key roles include:
- Enzymes: Catalyze biochemical reactions, significantly speeding up the rate of reactions. Examples include digestive enzymes like amylase and lipase.
- Structural proteins: Provide support and shape to cells and tissues. Examples include collagen in connective tissue and keratin in hair and nails.
- Transport proteins: Carry molecules across cell membranes or throughout the body. Examples include hemoglobin, which carries oxygen in the blood, and membrane transport proteins.
- Motor proteins: Generate movement, such as muscle contraction (e.g., actin and myosin) and intracellular transport.
- Hormones: Act as chemical messengers, regulating various physiological processes. Examples include insulin and growth hormone.
- Receptor proteins: Bind to specific molecules (ligands) and trigger cellular responses.
- Antibodies (immunoglobulins): Part of the immune system, recognizing and neutralizing foreign substances.
- Storage proteins: Store essential nutrients, such as ferritin (stores iron).
Amino Acid Metabolism: Synthesis, Degradation, and Essential Amino Acids
Our bodies can synthesize some amino acids, while others must be obtained from the diet. These latter are called essential amino acids. For humans, these are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. A balanced diet ensures sufficient intake of all essential amino acids for proper protein synthesis.
Amino acid metabolism encompasses both the synthesis and degradation of amino acids. Amino acid degradation releases nitrogenous waste products, primarily urea, which is excreted by the kidneys.
Conclusion: The Indispensable Role of Amino Acids in Life
Amino acids, the fundamental building blocks of proteins, are vital for all life processes. Their diverse structures and properties lead to the remarkable functional diversity of proteins. Understanding the structure, function, and metabolism of amino acids is essential for comprehending the complexities of biology and developing treatments for various diseases related to protein dysfunction. From the simplest enzyme to the most complex structural protein, amino acids are the cornerstone of life's intricate architecture. Further research continues to unveil the complexities of amino acids and their roles in health and disease, making it a vibrant and ever-evolving field of study. The continuing exploration of amino acid interactions and their roles in protein folding promises to uncover even more insights into the fundamental processes of life. The understanding of amino acids is not just a cornerstone of biochemistry, but a gateway to a deeper appreciation of the elegance and intricacy of biological systems.
Latest Posts
Latest Posts
-
Does The Hydrogen Molecule Obey The Octet Rule
Mar 18, 2025
-
How Is Atp Made During Fermentation
Mar 18, 2025
-
Is Main A Keyword In Fortran
Mar 18, 2025
-
How To Find Bond Dissociation Energy
Mar 18, 2025
-
Where Does Mrna Go After It Leaves The Nucleus
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Amino Acids Are The Monomeric Units Of Which Macromolecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
