Does The Hydrogen Molecule Obey The Octet Rule
Muz Play
Mar 18, 2025 · 6 min read
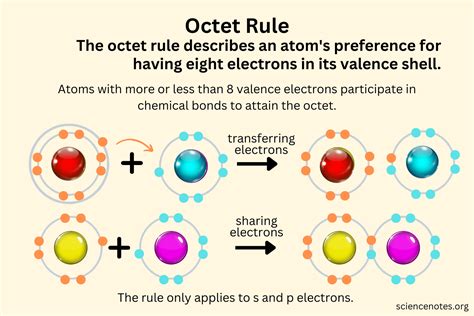
Table of Contents
Does the Hydrogen Molecule Obey the Octet Rule?
The octet rule, a cornerstone of basic chemistry, dictates that atoms tend to gain, lose, or share electrons to achieve a full outer electron shell of eight electrons, resembling the stable electron configuration of noble gases. This rule provides a simple framework for understanding chemical bonding and predicting molecular structures. However, its applicability isn't universally consistent, particularly with elements lighter than carbon, such as hydrogen. This article delves into the intricacies of hydrogen bonding, exploring whether the hydrogen molecule (H₂) adheres to the octet rule and examining the exceptions and limitations of this fundamental principle.
The Octet Rule: A Foundation, Not an Inflexible Law
The octet rule, while a useful guideline for predicting chemical behavior, isn't a strict law governing all chemical bonding. Many molecules and ions exist that deviate from the octet rule. These exceptions are crucial to understand to gain a complete picture of chemical bonding. The rule's effectiveness stems from the stability conferred by a filled valence shell, minimizing the overall energy of the atom. Atoms achieve this stable configuration through electron sharing (covalent bonds), electron transfer (ionic bonds), or remaining as stable, single atoms (noble gases).
Hydrogen's Unique Position: A Closer Look at its Electron Configuration
Hydrogen, with only one proton and one electron, occupies a unique position in the periodic table. Its single electron resides in the 1s orbital, the lowest energy level. Unlike heavier atoms with multiple electron shells and subshells, hydrogen only needs two electrons to completely fill its outermost shell – a duet rather than an octet. This fundamental difference underpins its departure from the conventional octet rule.
The Hydrogen Duet Rule: A More Applicable Description
Instead of adhering to the octet rule, hydrogen follows a duet rule, aiming for a stable configuration of two electrons in its valence shell. This is achieved by sharing electrons with another atom, forming a covalent bond. In the case of the hydrogen molecule (H₂), each hydrogen atom contributes its single electron to form a shared electron pair, fulfilling the duet rule for both atoms. This shared pair forms a single covalent bond, holding the two hydrogen atoms together.
The Hydrogen Molecule (H₂): A Detailed Analysis
The hydrogen molecule, the simplest diatomic molecule, is formed by the covalent bonding of two hydrogen atoms. Each atom shares its single electron with the other, creating a shared electron pair situated between the two nuclei. This electron pair effectively “belongs” to both hydrogen atoms, resulting in a stable, covalently bonded molecule.
Molecular Orbital Theory: A Deeper Understanding of Bonding in H₂
Molecular orbital theory (MOT) provides a more sophisticated explanation of bonding in H₂. The theory postulates that when two hydrogen atoms approach each other, their atomic orbitals combine to form molecular orbitals. These molecular orbitals encompass the entire molecule, rather than being localized on individual atoms. In H₂, the 1s atomic orbitals combine to form a bonding molecular orbital (σ1s) and an antibonding molecular orbital (σ*1s). The two electrons from the hydrogen atoms occupy the lower-energy bonding orbital, leading to a stable molecule. The antibonding orbital remains unoccupied.
Bond Order and Stability: Implications for Octet Rule
The bond order in H₂ is one, indicating a single covalent bond. This single bond signifies the electron sharing that fulfills the duet rule for each hydrogen atom. The stability of the H₂ molecule arises from the decrease in overall energy due to this electron sharing. Attempting to force the hydrogen molecule to obey the octet rule is not only inaccurate but also fails to account for its observed stability and reactivity.
Exceptions to the Octet Rule: Hydrogen and Beyond
Many molecules and ions deviate from the octet rule. These exceptions are broadly categorized into:
- Electron-deficient molecules: These molecules have fewer than eight electrons in their valence shell, often featuring elements like boron or beryllium.
- Electron-rich molecules: These molecules possess more than eight electrons in their valence shell, frequently involving elements in the third period or beyond, such as phosphorus or sulfur. This is possible because of the availability of d-orbitals.
- Odd-electron molecules (free radicals): These molecules contain an odd number of electrons, resulting in an unpaired electron. This often leads to high reactivity.
Hydrogen's case represents a distinct kind of exception: it doesn't attempt to achieve an octet but rather a duet, representing a fundamental difference in its electronic structure compared to heavier elements.
Comparing Hydrogen Bonding to Other Types of Bonding
Understanding how hydrogen bonding differs from other bonding types illuminates its unique nature and its departure from the octet rule.
Covalent Bonding vs Hydrogen Bonding
Covalent bonding involves the sharing of electron pairs between atoms to achieve stable electron configurations, fulfilling the octet or duet rule. This differs fundamentally from hydrogen bonding, which is a comparatively weaker intermolecular force of attraction between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. Hydrogen bonds are crucial for many properties of water and biological molecules. However, it's not a form of bonding that directly relates to the octet rule in the context of a single molecule.
Ionic Bonding: Electron Transfer, Not Sharing
Ionic bonding involves the complete transfer of electrons between atoms, resulting in the formation of oppositely charged ions that attract each other. This contrasts sharply with the electron sharing observed in covalent bonds, including those in the hydrogen molecule. Ionic bonds typically involve metals and nonmetals, and again, it doesn’t address the octet or duet rule in the same way.
Significance of Understanding Hydrogen's Behavior
Understanding that hydrogen follows the duet rule rather than the octet rule is crucial for several reasons:
- Accurate prediction of chemical behavior: It allows for more precise predictions regarding reactivity and bonding patterns of hydrogen-containing compounds.
- Correct interpretation of molecular structures: It enables correct interpretations of molecular geometries and bonding characteristics.
- Development of advanced theories: It forms a basis for the development and refinement of more sophisticated theories of chemical bonding, such as molecular orbital theory.
Conclusion: The Duet Rule Reigns Supreme for Hydrogen
In conclusion, the hydrogen molecule (H₂) does not obey the octet rule. Instead, it adheres to the duet rule, achieving stability by sharing a pair of electrons between its two hydrogen atoms. This forms a single covalent bond, fulfilling the requirement of two electrons in the valence shell of each hydrogen atom. While the octet rule serves as a valuable guideline for understanding chemical bonding in many instances, it’s crucial to acknowledge and understand its limitations, particularly regarding lighter elements like hydrogen. The duet rule, a more accurate and applicable principle in this context, effectively explains the stability and bonding behavior of the simplest of all diatomic molecules – the hydrogen molecule. Recognizing this distinction is fundamental to a comprehensive understanding of chemical bonding and the diverse behavior of chemical substances.
Latest Posts
Latest Posts
-
What Are Polymers Of Amino Acids
Mar 18, 2025
-
Shear Force And Bending Moment Diagram For Cantilever Beam
Mar 18, 2025
-
What Is The Percentage Of Truth In A Joke
Mar 18, 2025
-
T Test Formula For Dependent Samples
Mar 18, 2025
-
Eriksons Stage Of Integrity Vs Despair
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Does The Hydrogen Molecule Obey The Octet Rule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
