What Are Polymers Of Amino Acids
Muz Play
Mar 18, 2025 · 7 min read
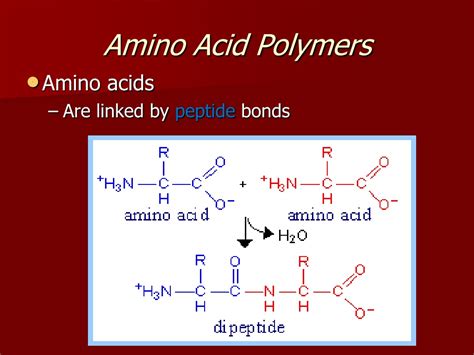
Table of Contents
What Are Polymers of Amino Acids? Delving into the World of Proteins
Proteins, the workhorses of life, are the subject of intense scientific scrutiny. Understanding their structure and function is crucial to comprehending the complexities of biological systems. At the heart of this understanding lies the fundamental question: what are polymers of amino acids? The simple answer is: proteins. However, to truly grasp the significance of this statement, we need to delve deeper into the fascinating world of amino acids, peptide bonds, protein structure, and their diverse roles in living organisms.
Understanding Amino Acids: The Building Blocks of Life
Amino acids are the fundamental monomers that constitute proteins. These organic molecules possess a central carbon atom (the alpha carbon) bonded to four groups:
- An amino group (-NH2): This group is basic and readily accepts a proton (H+).
- A carboxyl group (-COOH): This group is acidic and readily donates a proton (H+).
- A hydrogen atom (-H): A simple hydrogen atom.
- A side chain (R group): This is a variable group that differentiates the 20 standard amino acids. The R group's properties (size, charge, polarity, etc.) significantly influence the protein's overall structure and function.
This diversity in R groups gives rise to the 20 standard amino acids, each with unique characteristics. These amino acids are broadly categorized as:
-
Nonpolar, aliphatic amino acids: These amino acids have hydrophobic (water-repelling) side chains, often containing hydrocarbon groups. Examples include glycine, alanine, valine, leucine, isoleucine, and methionine.
-
Aromatic amino acids: These amino acids possess aromatic rings in their side chains. They are generally hydrophobic, with the exception of tyrosine, which can participate in hydrogen bonding. Examples include phenylalanine, tyrosine, and tryptophan.
-
Polar, uncharged amino acids: These amino acids have hydrophilic (water-attracting) side chains that can form hydrogen bonds. Examples include serine, threonine, cysteine, asparagine, and glutamine.
-
Positively charged (basic) amino acids: These amino acids have positively charged side chains at physiological pH. Examples include lysine, arginine, and histidine.
-
Negatively charged (acidic) amino acids: These amino acids have negatively charged side chains at physiological pH. Examples include aspartic acid and glutamic acid.
Peptide Bonds: Linking Amino Acids Together
The formation of a protein begins with the joining of amino acids through a process called peptide bond formation. This crucial step involves a dehydration reaction, where a water molecule is removed, linking the carboxyl group of one amino acid to the amino group of another. This creates a peptide bond, also known as an amide bond.
The resulting molecule is a dipeptide (two amino acids joined). Further addition of amino acids leads to the formation of oligopeptides (a few amino acids) and ultimately polypeptides (many amino acids linked together). Polypeptides are essentially the polymers of amino acids, representing the primary structure of a protein.
Protein Structure: From Primary to Quaternary
The three-dimensional structure of a protein is crucial for its function. This structure is hierarchical, comprising four levels of organization:
1. Primary Structure: The Amino Acid Sequence
The primary structure of a protein is simply the linear sequence of amino acids. This sequence is determined by the genetic code within DNA. Even a slight change in this sequence (e.g., a single amino acid substitution) can drastically alter the protein's properties and function. This is exemplified by sickle cell anemia, caused by a single amino acid substitution in the hemoglobin protein.
2. Secondary Structure: Local Folding Patterns
The secondary structure refers to the local folding patterns of the polypeptide chain stabilized by hydrogen bonds between the amino and carboxyl groups within the peptide backbone. Common secondary structure elements include:
- Alpha-helices: A right-handed coiled structure stabilized by hydrogen bonds between every fourth amino acid.
- Beta-sheets: Extended polypeptide chains arranged side-by-side, stabilized by hydrogen bonds between adjacent strands. These can be parallel or antiparallel, depending on the direction of the strands.
- Turns and loops: Short segments connecting alpha-helices and beta-sheets, contributing to the overall protein shape.
3. Tertiary Structure: The Overall 3D Arrangement
The tertiary structure represents the overall three-dimensional arrangement of a single polypeptide chain. This structure is stabilized by a variety of interactions between the amino acid side chains, including:
- Disulfide bridges: Covalent bonds between cysteine residues.
- Hydrophobic interactions: Clustering of nonpolar side chains in the protein's interior.
- Hydrogen bonds: Between polar side chains.
- Ionic interactions: Between oppositely charged side chains (salt bridges).
The tertiary structure dictates the protein's function. For example, the precise arrangement of amino acid side chains in the active site of an enzyme is essential for its catalytic activity.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains (subunits) assembled together to form a functional complex. This arrangement is known as the quaternary structure. The subunits are held together by similar types of interactions seen in tertiary structures. Hemoglobin, for instance, is a tetramer comprising four polypeptide subunits.
Functions of Proteins: A Diverse Array of Roles
Proteins perform an incredibly diverse range of functions within living organisms. Their versatility stems from their complex three-dimensional structures and the wide array of chemical properties conferred by their constituent amino acids. Some key functions include:
-
Enzymes: Catalyze biochemical reactions, accelerating their rate. Examples include digestive enzymes like amylase and proteases.
-
Structural proteins: Provide support and shape to cells and tissues. Examples include collagen (in connective tissues) and keratin (in hair and nails).
-
Transport proteins: Carry molecules across cell membranes or throughout the body. Examples include hemoglobin (carrying oxygen) and membrane transport proteins.
-
Motor proteins: Generate movement. Examples include myosin (in muscle contraction) and kinesin (in intracellular transport).
-
Hormones: Act as chemical messengers, coordinating cellular activities. Examples include insulin and growth hormone.
-
Antibodies: Part of the immune system, defending the body against foreign invaders.
-
Receptors: Receive and transduce signals from the environment.
Factors Affecting Protein Structure and Function
Several factors can influence a protein's structure and consequently its function:
-
pH: Changes in pH can alter the charge of amino acid side chains, disrupting ionic interactions and affecting protein folding.
-
Temperature: High temperatures can disrupt weak interactions (hydrogen bonds, hydrophobic interactions), leading to protein denaturation (loss of structure and function).
-
Salt concentration: High salt concentrations can also disrupt interactions between charged amino acid side chains.
-
Reducing agents: These agents can break disulfide bonds, altering protein structure.
-
Chaperones: Molecular chaperones assist in the correct folding of proteins, preventing aggregation and misfolding.
Studying Polymers of Amino Acids: Techniques and Applications
Investigating the structure and function of proteins employs various sophisticated techniques:
-
X-ray crystallography: Determines the three-dimensional structure of proteins by analyzing the diffraction patterns of X-rays passing through protein crystals.
-
Nuclear magnetic resonance (NMR) spectroscopy: Provides information on the protein structure and dynamics in solution.
-
Mass spectrometry: Determines the mass and composition of proteins and peptides.
-
Chromatography: Separates and purifies proteins based on their properties (size, charge, hydrophobicity).
-
Electrophoresis: Separates proteins based on their size and charge.
These techniques are crucial for advancing our understanding of protein structure-function relationships, with implications for medicine, biotechnology, and materials science. Understanding protein structure allows for the design of drugs that target specific proteins, the engineering of proteins with novel functions, and the development of new materials based on protein properties.
Conclusion: The Ever-Expanding World of Proteins
In conclusion, polymers of amino acids are proteins—complex biological macromolecules with diverse structures and functions. From catalyzing biochemical reactions to providing structural support, proteins are essential for life. Continued research into their structure, function, and interactions will undoubtedly unveil further insights into the complexities of biological systems and pave the way for advancements in various fields. The study of these remarkable molecules remains a vibrant and crucial area of scientific inquiry, constantly revealing new discoveries and applications. The more we understand about the intricacies of protein structure and function, the better equipped we are to address numerous challenges in medicine, biotechnology, and materials science.
Latest Posts
Latest Posts
-
Is Water A Mixture Or Pure Substance
Mar 18, 2025
-
Which Base Is Not Found In Rna
Mar 18, 2025
-
So Long To Pinky Here Comes The Thumb
Mar 18, 2025
-
When Two Amino Acids Are Joined Together
Mar 18, 2025
-
What Is The Difference Between Chemical Reaction And Nuclear Reaction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Are Polymers Of Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
