Are Covalent Bonds Between Two Nonmetals
Muz Play
Mar 15, 2025 · 6 min read
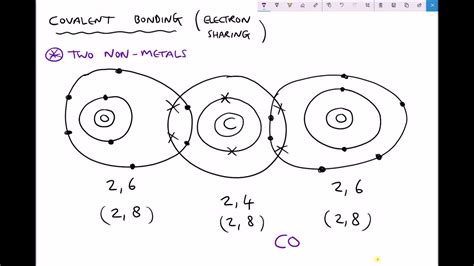
Table of Contents
Are Covalent Bonds Between Two Nonmetals? A Deep Dive into Chemical Bonding
Covalent bonds are a fundamental concept in chemistry, representing the strong attractive force holding atoms together in molecules. A common misconception is that covalent bonds only occur between nonmetals. While it's true that the vast majority of covalent bonds involve nonmetals, the reality is more nuanced. This article delves into the intricacies of covalent bonding, exploring why nonmetals are favored partners in this type of bonding, while also examining exceptions and nuances.
Understanding Covalent Bonds: A Shared Electron Affair
At the heart of a covalent bond lies the sharing of electrons between two atoms. Unlike ionic bonds, where electrons are transferred from one atom to another, covalent bonds involve a more collaborative approach. Atoms achieve stability, often fulfilling the octet rule (eight electrons in their valence shell), by sharing electrons to complete their outermost electron shell.
This sharing creates a strong attractive force between the positively charged nuclei of the atoms and the negatively charged shared electrons. This force is what constitutes the covalent bond. The strength of the bond depends on factors like the electronegativity of the atoms involved and the number of electron pairs shared.
Why Nonmetals are Favored Partners
Nonmetals typically have high electronegativities. Electronegativity is the measure of an atom's ability to attract electrons towards itself in a chemical bond. Since nonmetals have a strong attraction for electrons, they are less likely to donate electrons completely to another atom (as happens in ionic bonding). Instead, they prefer to share electrons to achieve a stable electron configuration.
Consider the example of a water molecule (H₂O). Both hydrogen and oxygen are nonmetals. Oxygen, with six valence electrons, needs two more to complete its octet. Hydrogen, with one valence electron, needs one more to achieve a stable duet (two electrons in its outermost shell). By sharing electrons, both atoms achieve stability: oxygen shares two electrons (one with each hydrogen), and each hydrogen shares its one electron with oxygen.
Strong electronegativity differences between two atoms usually lead to ionic bonds, not covalent bonds. The stronger the electronegativity difference, the more likely electron transfer is to occur. Nonmetals, however, generally exhibit similar electronegativities, making electron sharing a more energetically favorable option than complete electron transfer.
Types of Covalent Bonds: Exploring the Spectrum
Covalent bonds aren't all created equal. They exist on a spectrum, ranging from purely covalent to polar covalent, with varying degrees of electron sharing.
1. Purely Covalent Bonds: Equal Sharing
In purely covalent bonds, the electrons are shared equally between the two atoms. This occurs when the atoms have identical or very similar electronegativities. The classic example is a diatomic molecule like H₂, where the two hydrogen atoms share a pair of electrons equally. There's no significant charge separation across the bond.
2. Polar Covalent Bonds: Unequal Sharing
Polar covalent bonds represent an unequal sharing of electrons. This arises when there's a significant difference in electronegativity between the two atoms. The more electronegative atom attracts the shared electrons more strongly, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom.
Water (H₂O) is a perfect example of a molecule with polar covalent bonds. Oxygen is much more electronegative than hydrogen. Consequently, the shared electrons spend more time closer to the oxygen atom, resulting in a slightly negative charge on the oxygen and slightly positive charges on the hydrogens. This polarity is crucial for water's unique properties.
3. Coordinate Covalent Bonds: A Donation
Coordinate covalent bonds, also known as dative bonds, are a special type of covalent bond where one atom donates both electrons to the shared pair. This often happens when one atom has a lone pair of electrons and another atom has an empty orbital that can accommodate them. A common example is found in the ammonium ion (NH₄⁺), where the nitrogen atom donates a lone pair to a hydrogen ion (H⁺).
Exceptions and Nuances: When the Rules Bend
While nonmetals are the primary players in covalent bonding, some exceptions exist, blurring the lines between covalent and other bond types.
Covalent Bonds with Metalloids: A Gray Area
Metalloids, elements with properties intermediate between metals and nonmetals, can sometimes participate in covalent bonding. Silicon (Si), a metalloid, forms covalent bonds in compounds like silicon dioxide (SiO₂), the main component of sand. The bonding in these compounds is predominantly covalent, reflecting the metalloid's intermediate electronegativity.
Polarity and Bond Strength: A Complex Relationship
The polarity of a covalent bond influences its strength, though not always in a straightforward way. While generally, strong electronegativity differences lead to stronger ionic bonds, the relationship between polarity and covalent bond strength is more complex and depends on other factors like the size and number of atoms involved.
Multiple Bonds: Sharing More Than One Pair
Atoms can share more than one pair of electrons, forming multiple bonds. Double bonds (two shared pairs) and triple bonds (three shared pairs) are common. These multiple bonds are typically shorter and stronger than single bonds due to the increased electron density between the atoms. Nitrogen gas (N₂), with its triple bond, is a testament to the strength of multiple bonds.
Identifying Covalent Bonds: Practical Considerations
Identifying covalent bonds often involves considering the positions of elements on the periodic table. Nonmetals are generally located on the right side of the periodic table. However, as we've seen, the presence of metalloids and the nuances of bond polarity require careful consideration.
Beyond the Basics: Applications and Significance
Covalent bonds are essential to the existence of numerous substances vital to life and industry. Here are some key applications:
-
Organic Chemistry: The vast majority of organic compounds rely on covalent bonds to form the backbone of their molecules. From simple hydrocarbons to complex biomolecules like proteins and DNA, covalent bonding underpins the structure and function of organic matter.
-
Materials Science: Covalent bonding is crucial in developing advanced materials with specific properties. For example, the strong covalent bonds in diamonds contribute to their exceptional hardness, making them valuable in cutting and drilling tools.
-
Polymer Science: Polymers, long chains of repeating molecular units, are held together by covalent bonds. These materials have a wide range of applications, from plastics and rubbers to high-performance fibers.
Conclusion: A Dynamic World of Chemical Bonding
Covalent bonds, predominantly formed between nonmetals, represent a critical aspect of chemical bonding. Understanding the nuances of electron sharing, bond polarity, and the exceptions to the "nonmetal rule" is essential for comprehending the diverse properties and behavior of molecules. From the simple water molecule to complex biomolecules and advanced materials, covalent bonds are the fundamental building blocks that shape our world. The intricate interplay of electronegativity, bond strength, and multiple bonds reveals the dynamic and fascinating nature of this crucial chemical interaction. This deep understanding of covalent bonding allows us to predict and control the behavior of matter, leading to advancements in various scientific and technological fields.
Latest Posts
Latest Posts
-
What Elemsnts Are Most Likey To Turn Into Anions Why
Mar 15, 2025
-
What Is The Difference Between Hunger And Appetite
Mar 15, 2025
-
Boiling Point On Graph In Celsius
Mar 15, 2025
-
List The Classification Levels From Broadest To Most Specific
Mar 15, 2025
-
Equipments For Measuring Volume Of Acids
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Are Covalent Bonds Between Two Nonmetals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
