What Elemsnts Are Most Likey To Turn Into Anions Why
Muz Play
Mar 15, 2025 · 5 min read
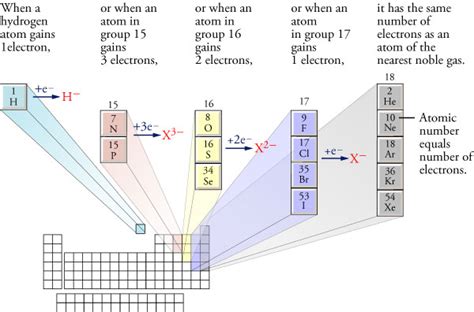
Table of Contents
What Elements Are Most Likely to Turn into Anions? Why?
Understanding why certain elements readily form anions—negatively charged ions—is fundamental to grasping chemical bonding and reactivity. This article delves deep into the atomic properties that predispose elements to gain electrons and become anions, exploring the periodic trends and exceptions that govern this behavior.
The Role of Electron Configuration
At the heart of anion formation lies the element's electron configuration. Atoms strive for stability, often achieving this by attaining a full outermost electron shell (valence shell), mirroring the stable electron configuration of noble gases. This principle, known as the octet rule, dictates that atoms tend to gain, lose, or share electrons to achieve eight electrons in their valence shell. Exceptions exist, particularly for elements with low atomic numbers.
Valence Electrons: The Key Players
The number of electrons in an atom's outermost shell—its valence electrons—directly impacts its propensity to form anions. Elements with a nearly full valence shell require relatively little energy to gain electrons and complete their octet, making anion formation energetically favorable.
Electronegativity: The Pulling Power
Electronegativity, a measure of an atom's ability to attract electrons in a chemical bond, plays a crucial role. Highly electronegative elements have a strong tendency to pull electrons towards themselves, making them more likely to accept electrons and form anions. This property increases across a period (left to right) on the periodic table and generally decreases down a group (top to bottom).
Periodic Trends in Anion Formation
The periodic table serves as a roadmap for predicting anion formation. Several trends are observable:
Group 17 (Halogens): The Anion Kings
The halogens (fluorine, chlorine, bromine, iodine, and astatine) are renowned for their exceptional ability to form anions. They possess seven valence electrons, needing only one more electron to achieve a stable octet. Their high electronegativity further enhances their electron-grabbing capabilities. Consequently, halogens readily form halide ions (F⁻, Cl⁻, Br⁻, I⁻, At⁻), making them quintessential examples of anion-forming elements.
Example: Chlorine (Cl) readily accepts an electron to form the chloride ion (Cl⁻), achieving a stable electron configuration similar to argon.
Group 16 (Chalcogens): A Strong Contender
The chalcogens (oxygen, sulfur, selenium, tellurium, and polonium) are another group prone to anion formation. With six valence electrons, they need two electrons to complete their octet. While less electronegative than halogens, they still readily accept electrons, forming anions with a -2 charge (e.g., O²⁻, S²⁻). Oxygen, in particular, is highly abundant and plays a vital role in numerous chemical reactions, often forming oxide anions (O²⁻).
Group 15 (Pnictogens): Less Frequent, but Still Possible
Pnictogens (nitrogen, phosphorus, arsenic, antimony, and bismuth) have five valence electrons. Gaining three electrons to form a stable octet is less energetically favorable compared to halogens and chalcogens. As a result, pnictogen anions are less common, but they can form under specific conditions, typically with highly electropositive metals. The resulting anions usually carry a -3 charge (e.g., N³⁻, P³⁻).
Beyond Group 15: Decreasing Anion Formation
Groups to the left of Group 15 (Groups 1-14) generally have lower electronegativities and a smaller tendency to form anions. While some elements in these groups can form anions under specific circumstances, it's far less prevalent than in groups 16 and 17.
Factors Affecting Anion Stability
While the periodic trends provide a good general guide, other factors can influence anion stability:
Ionic Radius: Size Matters
As the ionic radius increases (moving down a group), the negative charge is spread over a larger volume, reducing the charge density. This makes larger anions less stable than smaller ones because the electron-electron repulsion is lessened. For example, iodide (I⁻) is larger and less stable than fluoride (F⁻).
Electron-Electron Repulsion: A Balancing Act
The more electrons an anion possesses, the greater the electron-electron repulsion within the ion. This repulsion can destabilize larger anions, making them less favorable. This is one reason why pnictogen anions (-3 charge) are less common than chalcogen anions (-2 charge).
Polarizability: The Influence of External Fields
Anion polarizability, the ease with which the electron cloud can be distorted by an external electric field, influences anion stability. Highly polarizable anions are more susceptible to interaction with other ions or molecules, affecting their overall stability.
Exceptions and Special Cases
While the octet rule provides a useful framework, it is not universally applicable. Exceptions arise, particularly with elements that violate the octet rule:
Transition Metals: Complex Anion Formation
Transition metals, with their partially filled d orbitals, often form complex anions with variable oxidation states. These anions frequently involve coordination complexes, where the transition metal ion is surrounded by ligands (neutral molecules or ions).
Lanthanides and Actinides: Unique Behavior
The electron configurations of lanthanides and actinides are complex, often deviating from the typical octet rule. Their behavior in anion formation is less predictable and requires more specialized analysis.
Conclusion
The formation of anions is driven primarily by the quest for electronic stability, guided by the atom's electron configuration, electronegativity, and periodic trends. While halogens and chalcogens are the most prominent anion-forming elements, exceptions and variations arise, emphasizing the complexity of chemical behavior and the interplay of various atomic properties. Understanding these factors is crucial for predicting reactivity, designing chemical reactions, and interpreting the properties of ionic compounds. This detailed exploration has revealed the intricacies involved, providing a comprehensive overview of what makes some elements more likely to become anions than others. The interplay of electron configuration, electronegativity, ionic size, and electron-electron repulsion ultimately governs this fundamental aspect of chemical behavior.
Latest Posts
Latest Posts
-
Period 2 On The Periodic Table
Mar 15, 2025
-
Heat Is A Measure Of The Random Of Molecules
Mar 15, 2025
-
A Vector Has Magnitude And Direction
Mar 15, 2025
-
What Elemsnt Are Most Likey To Turn Into Anions Why
Mar 15, 2025
-
Basic Unit Of Structure And Function In An Organism
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Elemsnts Are Most Likey To Turn Into Anions Why . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
