Are Hydrophobic Interactions Stronger Than Hydrogen Bonds
Muz Play
Mar 17, 2025 · 6 min read
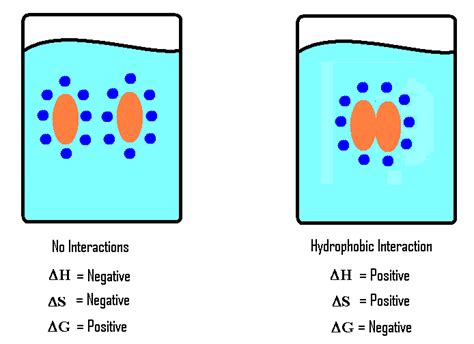
Table of Contents
Are Hydrophobic Interactions Stronger Than Hydrogen Bonds? A Deep Dive into Intermolecular Forces
The question of whether hydrophobic interactions are stronger than hydrogen bonds is a complex one, lacking a simple yes or no answer. The strength of these intermolecular forces depends heavily on the specific context, including the molecules involved, the surrounding environment (e.g., solvent), and temperature. While hydrogen bonds are individually stronger, hydrophobic interactions can collectively exert a more substantial influence, particularly in aqueous solutions. Let's delve into the intricacies of both forces to understand their comparative strengths and the situations where one dominates over the other.
Understanding Hydrogen Bonds
Hydrogen bonds are a special type of dipole-dipole attraction between molecules, not a covalent bond. They occur when a hydrogen atom covalently bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a different molecule. This creates a strong electrostatic interaction. The strength of a hydrogen bond is typically in the range of 5 to 30 kJ/mol, significantly weaker than covalent bonds but stronger than many other intermolecular forces.
Key Characteristics of Hydrogen Bonds:
- Specificity: Hydrogen bonds are directional; they form preferentially along the axis of the electronegative atom's lone pair. This contributes to their strength and specificity in biological systems, influencing protein folding and DNA structure.
- Strength Variation: The strength of a hydrogen bond varies depending on the electronegativity of the atoms involved, the geometry of the interacting molecules, and the presence of other competing interactions. A hydrogen bond between water molecules, for example, is relatively strong compared to one involving a less electronegative atom.
- Importance in Biological Systems: Hydrogen bonds are crucial for the structure and function of many biological molecules. They stabilize protein secondary structures (alpha-helices and beta-sheets), hold DNA strands together, and are vital for enzyme-substrate interactions.
Decoding Hydrophobic Interactions
Unlike hydrogen bonds, hydrophobic interactions are not a direct attractive force between molecules. Instead, they are driven by the tendency of nonpolar molecules to aggregate in aqueous solutions to minimize their contact with water. Water molecules, being polar, form strong hydrogen bonds with each other. The presence of a nonpolar molecule disrupts this hydrogen bonding network, causing a decrease in entropy. To maximize entropy (and minimize the disruption to the water structure), nonpolar molecules cluster together, effectively excluding water from the core of the aggregate.
The Entropic Drive Behind Hydrophobic Interactions:
The driving force behind hydrophobic interactions is entropic, not enthalpic. This means it's primarily about maximizing disorder or randomness, not minimizing energy. The clustering of nonpolar molecules increases the entropy of the surrounding water molecules, outweighing the small decrease in entropy associated with the aggregation of the nonpolar molecules themselves.
Strength and Context Dependency of Hydrophobic Interactions:
The "strength" of a hydrophobic interaction is difficult to quantify directly because it's not a single bond but a collective effect. However, the free energy change associated with hydrophobic interactions can be substantial, often exceeding the strength of individual hydrogen bonds when many nonpolar molecules are involved. The effective strength of hydrophobic interactions typically ranges from 3 to 12 kJ/mol per CH2 group, but this can increase significantly with increasing surface area of the interacting molecules. This is highly context-dependent and influenced by factors like temperature, pressure and the presence of other solutes.
Comparing the Two: Strength in Numbers vs. Individual Power
A single hydrogen bond is generally stronger than the contribution of a single CH2 group to a hydrophobic interaction. However, hydrophobic interactions often involve numerous nonpolar groups interacting collectively. This cumulative effect can lead to a significant overall free energy change, making the net interaction stronger than a small number of individual hydrogen bonds.
Consider a protein folding in an aqueous solution: hydrogen bonds contribute to the secondary structure (alpha-helices and beta-sheets), but hydrophobic interactions are crucial for driving the overall folding process. The burial of hydrophobic amino acid side chains within the protein core maximizes entropy of the surrounding water molecules, leading to a stable, compact protein structure. This process is significantly influenced by the large number of hydrophobic interactions involved.
Factors Influencing the Balance: Temperature, Pressure, and Solvents
The relative importance of hydrogen bonds and hydrophobic interactions is not static. Several environmental factors can shift the balance:
Temperature:
Increased temperature weakens both hydrogen bonds and hydrophobic interactions. However, the effect is usually more pronounced on hydrogen bonds, as higher temperatures increase the kinetic energy of molecules, disrupting the delicate hydrogen bond network more readily. Hydrophobic interactions, although weakened, might still play a significant role at elevated temperatures due to their cumulative nature.
Pressure:
Pressure can influence both interactions but in different ways. High pressure generally favors compact structures, benefiting hydrophobic interactions that promote aggregation. The effect on hydrogen bonds is more nuanced and depends on the system.
Solvents:
The solvent plays a crucial role. In aqueous solutions, hydrophobic interactions are dominant due to the strong hydrogen bonding network of water. However, in nonpolar solvents, hydrophobic interactions are diminished because nonpolar molecules have no energetic penalty for interacting with the solvent. Hydrogen bonds might still be relevant if polar groups are present on the molecules.
Biological Implications and Examples
The interplay between hydrogen bonds and hydrophobic interactions is central to many biological phenomena:
- Protein Folding: Hydrophobic interactions drive the initial collapse of the protein chain, while hydrogen bonds stabilize secondary and tertiary structures.
- Membrane Formation: Lipid bilayers, the basis of cell membranes, are stabilized by hydrophobic interactions between the fatty acid tails, while hydrogen bonds play a role in the interactions of polar head groups with water.
- Enzyme-Substrate Interactions: Both hydrogen bonds and hydrophobic interactions can contribute to the binding of a substrate to an enzyme's active site, often working synergistically.
- DNA Structure: Hydrogen bonds are essential for maintaining the double helix structure of DNA, while hydrophobic interactions play a role in base stacking.
Conclusion: It's a Context-Dependent Dance
In conclusion, there's no straightforward answer to whether hydrophobic interactions are stronger than hydrogen bonds. A single hydrogen bond might be stronger than a single contribution to a hydrophobic interaction. However, the cumulative effect of numerous hydrophobic interactions often results in a more significant free energy change, outweighing the contribution from a smaller number of individual hydrogen bonds in certain situations. The outcome is strongly context-dependent and influenced by temperature, pressure, and the nature of the solvent and molecules involved. Both forces play crucial and complementary roles in numerous biological and chemical processes, often working together to shape the structure and function of molecules and systems. The interplay between them is a complex and fascinating area of ongoing research. Understanding their relative contributions is vital to comprehending the behavior of molecules in different environments.
Latest Posts
Latest Posts
-
Function Of A Stage On A Microscope
Mar 17, 2025
-
What Is The Net Gain Of Atp In Glycolysis
Mar 17, 2025
-
Which Is Bigger Tert Or Chlorine
Mar 17, 2025
-
A Supersaturated Solution Is One That
Mar 17, 2025
-
Transitive Closure For Binary Relation Definition
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Are Hydrophobic Interactions Stronger Than Hydrogen Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
