Which Is Bigger Tert Or Chlorine
Muz Play
Mar 17, 2025 · 5 min read
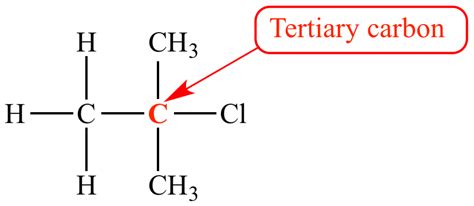
Table of Contents
Which is Bigger: Tert-Butyl or Chlorine? A Deep Dive into Atomic and Molecular Size
Determining which is "bigger" between a tert-butyl group and a chlorine atom requires a nuanced understanding of atomic and molecular size. It's not a simple comparison of single values, but rather a consideration of different aspects of size depending on the context. This article delves into the complexities of comparing the sizes of these two chemical entities, exploring their atomic radii, van der Waals radii, and the overall spatial occupancy of the tert-butyl group.
Understanding Atomic Size: Chlorine
Chlorine (Cl) is a halogen element found in Group 17 of the periodic table. Its atomic number is 17, meaning it has 17 protons and 17 electrons. The size of a chlorine atom is primarily determined by its atomic radius, which represents the average distance between the nucleus and the outermost electron shell.
Factors Affecting Chlorine's Atomic Radius:
- Nuclear Charge: The positive charge of the chlorine nucleus attracts the negatively charged electrons, pulling them closer and reducing the atomic radius.
- Electron Shielding: Inner electrons shield the outer electrons from the full effect of the nuclear charge. This shielding effect slightly counteracts the nuclear attraction, increasing the atomic radius.
- Electron-Electron Repulsion: Repulsion between electrons in the same shell increases the size of the atom.
Chlorine's atomic radius is relatively small compared to larger atoms due to the strong nuclear charge and relatively compact electron configuration. The effective nuclear charge experienced by the valence electrons is high, leading to a strong pull towards the nucleus.
Understanding Molecular Size: Tert-Butyl Group
The tert-butyl group (t-Bu or t-C₄H₉) is a branched alkyl group with the formula (CH₃)₃C−. It's not an atom, but a molecular fragment consisting of four carbon atoms and nine hydrogen atoms covalently bonded together in a specific tetrahedral arrangement. Therefore, defining its "size" requires a slightly different approach.
Defining the Size of the Tert-Butyl Group:
Several metrics can be used to assess the size of the tert-butyl group:
-
Van der Waals Radius: This describes the effective size of the molecule, considering the space occupied by its electron cloud. It's the distance of closest approach between two non-bonded atoms. Calculating the overall van der Waals radius of a molecule like tert-butyl is complex, involving the summation of individual atomic van der Waals radii and considering steric effects. However, it gives a good approximation of the space the molecule occupies.
-
Spatial Occupancy/Steric Bulk: This accounts for the overall volume the group occupies in three-dimensional space. The tert-butyl group is known for its significant steric bulk due to its branched structure. The three methyl groups project outwards, creating considerable spatial hindrance. This steric bulk greatly influences the reactivity and behavior of molecules containing a tert-butyl group.
Comparing Chlorine and Tert-Butyl: A Multifaceted Analysis
Directly comparing the atomic radius of chlorine to the overall size of the tert-butyl group is inherently problematic. However, we can make a comparative assessment by considering several factors:
1. Atomic Radius vs. Van der Waals Radius:
The atomic radius of chlorine is significantly smaller than the van der Waals radius of the tert-butyl group. This is primarily because the tert-butyl group encompasses a much larger volume of space due to its four carbon atoms and nine hydrogen atoms, each contributing to its overall van der Waals envelope.
2. Steric Hindrance and Spatial Occupancy:
The tert-butyl group exhibits considerable steric hindrance due to its branched structure. This makes it occupy significantly more space than a single chlorine atom. In chemical reactions, the tert-butyl group will often experience steric clashes, preventing reactions that might be accessible to smaller groups. Chlorine, being a single atom, has far less steric hindrance.
3. Context-Dependent Comparison:
The "bigger" entity depends on the context:
- Space Occupancy: The tert-butyl group undeniably occupies significantly more three-dimensional space than a single chlorine atom.
- Atomic/Molecular Interactions: In reactions involving steric effects, the tert-butyl group’s large size plays a dominant role. Chlorine's size is far less influential in these scenarios.
- Simple Comparison of Radii: If we're solely comparing atomic radii (for chlorine) and the approximate van der Waals radius of the tert-butyl group, the tert-butyl group will appear considerably larger.
4. Bonding and Reactivity:
The tert-butyl group's size significantly affects its reactivity. Its steric bulk often hinders nucleophilic substitutions and other reactions involving approaches from specific directions. Chlorine, being smaller and more easily accessible, demonstrates different reaction characteristics.
Visualizing the Difference:
Imagine a single chlorine atom as a small marble, while the tert-butyl group is akin to a slightly deflated beach ball. The beach ball (tert-butyl) clearly occupies much more space than the marble (chlorine). The tert-butyl group's bulk is a crucial factor in its chemical behavior and interaction with other molecules.
Conclusion: No Simple Answer
The question "which is bigger, tert-butyl or chlorine?" doesn't have a straightforward yes or no answer. The tert-butyl group, with its four carbon atoms and nine hydrogen atoms, occupies substantially more space than a single chlorine atom. However, the "size" we're considering must be carefully defined. If we are referring to atomic radius, chlorine is smaller. If we're evaluating spatial occupancy and steric hindrance, the tert-butyl group is considerably larger. The appropriate answer is always context-dependent, requiring a clear understanding of the specific chemical phenomenon being discussed. The significant steric effects of the tert-butyl group make its "size" a major factor in its chemical behavior and interactions. Therefore, considering both atomic and molecular size, and steric effects, the tert-butyl group is unequivocally larger in terms of spatial occupancy and steric bulk.
Latest Posts
Latest Posts
-
Hydrogen Is A Metal Or Nonmetal
Mar 17, 2025
-
How To Calculate The Instantaneous Rate Of A Reaction
Mar 17, 2025
-
Is Delta H Positive For Endothermic
Mar 17, 2025
-
Wo Letter Symbol From The Periodic Table
Mar 17, 2025
-
Periodic Table Solid Liquid And Gas
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Is Bigger Tert Or Chlorine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
