Is Delta H Positive For Endothermic
Muz Play
Mar 17, 2025 · 5 min read
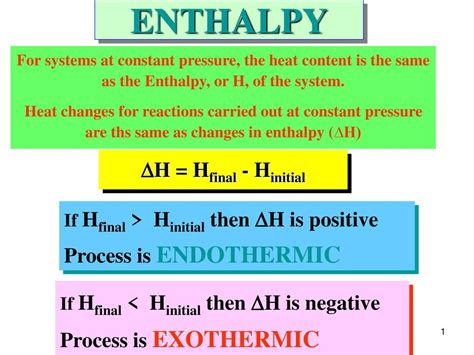
Table of Contents
Is ΔH Positive for Endothermic Reactions? A Deep Dive into Enthalpy
Understanding enthalpy changes (ΔH) is crucial for grasping the fundamentals of thermodynamics and chemical reactions. This article will explore the relationship between enthalpy and endothermic reactions, definitively answering the question: Is ΔH positive for endothermic reactions? We'll delve into the concepts, provide clear explanations, and explore practical examples to solidify your understanding.
Understanding Enthalpy (H)
Enthalpy is a thermodynamic property representing the total heat content of a system at constant pressure. It's a state function, meaning its value depends only on the system's current state, not on the path taken to reach that state. In simpler terms, enthalpy measures the total energy within a system, including its internal energy and the product of its pressure and volume.
We often express enthalpy changes (ΔH) rather than absolute enthalpy values. This change reflects the heat absorbed or released during a chemical or physical process. This change is crucial for determining whether a reaction is exothermic or endothermic.
The Significance of ΔH
The enthalpy change (ΔH) is a critical indicator of a reaction's energy profile. A negative ΔH signifies an exothermic reaction, where heat is released to the surroundings, resulting in a decrease in the system's enthalpy. Conversely, a positive ΔH indicates an endothermic reaction, where heat is absorbed from the surroundings, leading to an increase in the system's enthalpy.
Endothermic Reactions: A Detailed Look
Endothermic reactions are characterized by their absorption of heat from their surroundings. This heat absorption is necessary for the reaction to proceed, resulting in a net increase in the system's enthalpy. Think of it like this: the reactants need to absorb energy to overcome an energy barrier and transform into products.
Visualizing Endothermic Reactions with Energy Diagrams
Energy diagrams are invaluable tools for visualizing the energy changes involved in chemical reactions. For endothermic reactions, the energy diagram shows the products at a higher energy level than the reactants. The difference in energy levels represents the positive ΔH value.
[Insert a simple energy diagram showing reactants at a lower energy level, an activation energy hump, and products at a higher energy level. Clearly label ΔH as positive.]
Why is Heat Absorbed in Endothermic Reactions?
The absorption of heat in endothermic reactions is fundamentally linked to the breaking and forming of chemical bonds. In many cases, more energy is required to break the bonds in the reactants than is released when new bonds form in the products. This net energy difference is absorbed from the surroundings, leading to the characteristic positive ΔH.
Examples of Endothermic Reactions
Numerous examples of endothermic reactions occur in everyday life and within various scientific disciplines. Let's examine a few:
1. Photosynthesis: The Engine of Life
Photosynthesis, the process by which plants convert light energy into chemical energy, is a prime example of an endothermic reaction. Plants absorb sunlight to power the conversion of carbon dioxide and water into glucose and oxygen. This process requires a significant input of energy, resulting in a positive ΔH.
Equation: 6CO₂ + 6H₂O + Light Energy → C₆H₁₂O₆ + 6O₂
2. Melting Ice: A Phase Transition
The melting of ice into liquid water is another familiar endothermic process. Heat energy from the surroundings is absorbed by the ice, weakening the hydrogen bonds holding the water molecules in their rigid crystalline structure. This energy input leads to a phase change and a positive ΔH.
3. Dissolving Ammonium Nitrate: A Common Demonstration
Dissolving ammonium nitrate (NH₄NO₃) in water is a classic endothermic demonstration. The process absorbs heat from the surrounding water, resulting in a noticeable decrease in temperature. This cooling effect is due to the positive ΔH associated with the dissolution process.
4. Cooking an Egg: A Complex Endothermic Process
Cooking an egg involves various complex endothermic reactions. Heat from the pan is absorbed by the egg proteins, causing them to denature and change their structure. This transformation involves bond breaking and reformation, contributing to the overall positive ΔH of the cooking process.
ΔH and the First Law of Thermodynamics
The positive ΔH in endothermic reactions aligns perfectly with the First Law of Thermodynamics (the law of conservation of energy). This law states that energy cannot be created or destroyed, only transferred or transformed. In endothermic reactions, the energy absorbed from the surroundings is converted into the increased internal energy of the products, leading to the positive ΔH.
Factors Affecting ΔH in Endothermic Reactions
Several factors can influence the magnitude of ΔH in endothermic reactions:
- Nature of Reactants and Products: The types of chemical bonds involved significantly impact the energy change. Stronger bonds require more energy to break, leading to a larger positive ΔH.
- Temperature: Higher temperatures generally increase the kinetic energy of molecules, making it easier to overcome activation energy barriers, potentially influencing the overall ΔH.
- Pressure: Pressure changes can affect the reaction's equilibrium and, in turn, its enthalpy change. However, the effect is usually more pronounced in reactions involving gases.
Practical Applications of Endothermic Reactions
The understanding and application of endothermic reactions are vital across various fields:
- Refrigeration and Air Conditioning: Many refrigerants rely on endothermic processes to absorb heat from the surroundings, providing cooling.
- Industrial Processes: Several industrial processes utilize endothermic reactions to produce desired products, often requiring significant energy input.
- Chemical Synthesis: Understanding enthalpy changes is crucial for designing and optimizing chemical synthesis pathways.
Conclusion: Yes, ΔH is Positive for Endothermic Reactions
To reiterate the central theme of this article: Yes, ΔH is always positive for endothermic reactions. The positive enthalpy change signifies the absorption of heat from the surroundings, a defining characteristic of these reactions. Understanding this fundamental relationship is crucial for comprehending various chemical and physical processes, from the everyday melting of ice to the complex reactions driving life itself. By understanding enthalpy changes and the energy profiles of reactions, we gain valuable insights into the driving forces behind chemical transformations and their applications in various fields. The positive ΔH value is not merely a mathematical observation but a reflection of the fundamental energy transfer inherent in endothermic processes.
Latest Posts
Latest Posts
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
-
What Are The Functions Of The Family
Mar 18, 2025
-
Trends In The Periodic Table Melting Point
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Delta H Positive For Endothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
