Hydrogen Is A Metal Or Nonmetal
Muz Play
Mar 17, 2025 · 4 min read
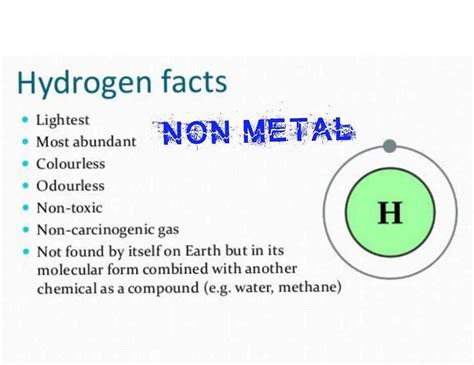
Table of Contents
Is Hydrogen a Metal or a Nonmetal? A Deep Dive into its Unique Properties
The seemingly simple question, "Is hydrogen a metal or a nonmetal?" belies a complex answer that delves into the fascinating world of chemistry and the periodic table. While hydrogen sits at the top of the periodic table, nestled amongst the alkali metals, its properties defy simple categorization. This article will explore the unique characteristics of hydrogen, examining its metallic and nonmetallic behaviors, and ultimately discussing why its classification remains a subject of ongoing scientific debate.
The Periodic Table Placement: A Starting Point
Hydrogen's position at the top of Group 1 (alkali metals) is often the source of initial confusion. Alkali metals are known for their highly reactive metallic properties. However, hydrogen's behavior deviates significantly from its group-mates. This discrepancy arises from its unique electronic configuration and its relatively small size. It possesses only one proton and one electron, making it the lightest element in existence.
Electronic Configuration and Chemical Reactivity
Hydrogen's single electron resides in the 1s orbital. This simple electronic structure influences its chemical behavior in two primary ways:
-
Electron Sharing (Covalent Bonding): Hydrogen often shares its single electron with other atoms, forming covalent bonds. This is a characteristic primarily associated with nonmetals. Examples include the covalent bonds in water (H₂O) and methane (CH₄). This electron-sharing behavior stands in contrast to the electron donation typical of alkali metals, which readily lose their valence electrons to form ionic bonds.
-
Electron Gain (Hydride Ion Formation): Under certain conditions, hydrogen can gain an electron to form a hydride ion (H⁻). This negatively charged ion is characteristic of nonmetals, which tend to gain electrons to achieve a stable electron configuration.
These contrasting behaviors highlight hydrogen's ambiguous nature, exhibiting characteristics traditionally associated with both metals and nonmetals.
Examining Hydrogen's Metallic and Nonmetallic Traits
Let's delve deeper into the specifics of hydrogen's metallic and nonmetallic tendencies:
Nonmetallic Characteristics:
-
Gaseous State at Room Temperature: Unlike most metals, which are solids at room temperature, hydrogen exists as a diatomic gas (H₂). This gaseous nature is typical of many nonmetals.
-
Poor Electrical and Thermal Conductivity: Metals are renowned for their excellent conductivity of both electricity and heat. Hydrogen, however, is a poor conductor of both, a characteristic aligning with nonmetals.
-
Low Melting and Boiling Points: Hydrogen's melting and boiling points are exceptionally low compared to metals. These low values are consistent with the weak intermolecular forces present in nonmetallic substances.
-
Formation of Covalent Compounds: As previously mentioned, hydrogen's tendency to form covalent bonds with other elements is a hallmark of nonmetals. It readily shares electrons to form numerous covalent compounds.
-
Non-lustrous Appearance: Unlike the characteristic luster of most metals, hydrogen gas is colorless and transparent.
Metallic Characteristics (under extreme conditions):
While under typical conditions hydrogen displays primarily nonmetallic behavior, under extreme pressure, it exhibits some metallic traits:
-
Metallic Hydrogen: A Theoretical Prediction: At extremely high pressures, exceeding millions of atmospheres, theoretical calculations predict a phase transition where hydrogen becomes a metallic solid. This metallic hydrogen would possess high electrical conductivity and other metallic properties.
-
Experimental Evidence and Challenges: While the theoretical prediction of metallic hydrogen is widely accepted, creating and observing this state in a laboratory setting has proven incredibly challenging. The immense pressures required are difficult to achieve and maintain, and the experimental evidence remains limited and contested.
Why the Classification Remains Debatable
The debate surrounding hydrogen's classification stems from its unique position as the lightest and simplest element. Its behavior varies greatly depending on conditions:
-
Context-Dependent Behavior: Hydrogen's properties are heavily influenced by temperature, pressure, and the chemical environment. This context-dependent behavior makes it difficult to assign it to a single, definitive category.
-
No Single Defining Property: There's no single property that definitively distinguishes metals from nonmetals. Hydrogen displays a mixture of traits typically associated with both.
-
Practical Considerations: For many practical applications, considering hydrogen's nonmetallic behavior is sufficient. Its use in chemical reactions and its behavior in compounds largely align with nonmetallic tendencies.
Conclusion: A Unique Element
Ultimately, while hydrogen is often treated as a nonmetal due to its prevalent nonmetallic properties under standard conditions, its potential to exhibit metallic characteristics under extreme pressure highlights its unique and complex nature. It doesn't perfectly fit into either the metal or nonmetal category, making it a truly exceptional element. The ongoing research into metallic hydrogen further underscores the complexity of this seemingly simple atom and its continued ability to challenge our understanding of elemental classifications. Its versatile nature makes it a crucial component in a vast array of chemical processes and fuels ongoing research in fields ranging from energy production to material science. The debate over its classification serves as a reminder of the nuances within the periodic table and the ongoing quest for a comprehensive understanding of the elements. Instead of forcing it into a rigid classification, it's perhaps more accurate to recognize hydrogen as a unique element that bridges the gap between metals and nonmetals, exhibiting properties characteristic of both depending on the prevailing conditions.
Latest Posts
Latest Posts
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
-
What Are The Functions Of The Family
Mar 18, 2025
-
Trends In The Periodic Table Melting Point
Mar 18, 2025
-
What Two Regions Make Up All Atoms
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Hydrogen Is A Metal Or Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
