Periodic Table Solid Liquid And Gas
Muz Play
Mar 17, 2025 · 6 min read
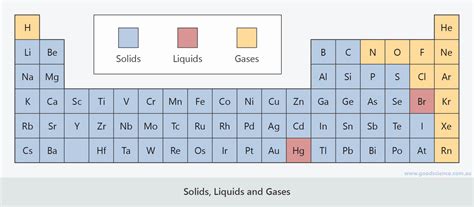
Table of Contents
The Periodic Table: A Solid, Liquid, and Gaseous Exploration
The periodic table, a cornerstone of chemistry, organizes chemical elements based on their atomic number, electron configuration, and recurring chemical properties. While often visualized as a static grid, the elements within exhibit dynamic behaviors, existing in various states of matter – solid, liquid, and gas – depending on temperature and pressure. Understanding these states and their relation to the periodic table provides crucial insight into the nature of matter and the diverse properties of the elements.
The Three Fundamental States of Matter: Solid, Liquid, and Gas
Before diving into the periodic table's relationship with these states, let's briefly review the characteristics of each:
Solids: The Rigid Structures
Solids are characterized by their strong intermolecular forces, holding their constituent particles (atoms, ions, or molecules) in a rigid, fixed structure. This results in a definite shape and volume. The particles vibrate in place, but their movement is restricted. Solids possess a high density due to the close packing of their particles. Examples include iron (Fe), diamond (C), and sodium chloride (NaCl).
Liquids: The Flowing Forms
Liquids exhibit weaker intermolecular forces than solids. Their particles are more mobile, allowing liquids to flow and take the shape of their container while maintaining a constant volume. Liquids have a moderate density and are relatively incompressible. Examples include water (H₂O), mercury (Hg), and ethanol (C₂H₅OH).
Gases: The Expansive Entities
Gases possess the weakest intermolecular forces, resulting in particles that are widely dispersed and move freely. Gases have neither a definite shape nor volume, expanding to fill their container completely. They are highly compressible and have a low density. Examples include oxygen (O₂), nitrogen (N₂), and helium (He).
The Periodic Table and States of Matter: Trends and Exceptions
The periodic table doesn't explicitly predict the state of an element at a specific temperature and pressure. However, it provides crucial clues about the underlying factors influencing an element's physical state. These factors are primarily:
-
Atomic Mass and Size: Heavier elements generally have stronger intermolecular forces, favoring a solid state at room temperature. Larger atoms may have weaker forces due to increased distance between the nuclei and valence electrons, potentially favoring a gaseous state.
-
Electronegativity and Bonding: Elements with high electronegativity tend to form strong ionic or covalent bonds, leading to solid states. Elements with low electronegativity may form weaker metallic bonds, potentially influencing melting and boiling points.
-
Intermolecular Forces: The strength of these forces (van der Waals forces, hydrogen bonding, dipole-dipole interactions) significantly impact the state of matter. Stronger forces favor solid states at lower temperatures.
Let's explore these trends across different groups and periods of the periodic table:
Group 18: The Noble Gases – Always Gaseous (Mostly)
The noble gases (Helium, Neon, Argon, Krypton, Xenon, and Radon) are uniquely positioned in the periodic table. Their full valence electron shells result in extremely weak intermolecular forces. Consequently, all noble gases exist as gases at room temperature and standard pressure. Only under extreme conditions of low temperature and high pressure can they be liquefied or solidified.
Group 1: The Alkali Metals – Solid, Reactive Metals
Alkali metals (Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium) are highly reactive metals existing as solids at room temperature due to their relatively strong metallic bonding. Their low electronegativity and ease of ionization explain their high reactivity.
Group 17: The Halogens – Diverse States
Halogens (Fluorine, Chlorine, Bromine, Iodine, and Astatine) show a clear trend in states of matter as you move down the group. Fluorine and Chlorine are gases, Bromine is a liquid, and Iodine is a solid at room temperature. This trend is directly related to the increasing strength of van der Waals forces with increasing atomic size and mass.
Group 14: Carbon’s Unique Solid Forms
Group 14 showcases carbon's remarkable ability to form various allotropes (different structural forms of the same element) with vastly different properties. Diamond is an extremely hard solid due to its strong covalent network structure, while graphite is a soft, layered solid. Fullerenes, another allotrope, also exist as solids.
Transition Metals: Mostly Solid, Highly Variable
The transition metals, residing in the central block of the periodic table, are mostly solids at room temperature due to their strong metallic bonding. However, their melting and boiling points vary significantly depending on the element's electronic configuration and other factors. Mercury (Hg) is a notable exception, being a liquid at room temperature due to its relatively weak metallic bonding.
Metalloids: A Bridge Between Metals and Nonmetals
Metalloids (Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium) display properties of both metals and nonmetals, reflecting their intermediate position in the periodic table. Many exist as solids at room temperature, with varying melting and boiling points.
Factors Affecting State Transitions
The transition between solid, liquid, and gaseous states is governed by temperature and pressure.
Temperature: The Energy Driver
Increasing temperature provides the kinetic energy necessary to overcome intermolecular forces. Heating a solid increases particle vibrations until the solid melts into a liquid. Further heating increases particle kinetic energy, eventually leading to vaporization (liquid to gas).
Pressure: The Constraining Force
Pressure influences the spacing between particles. Increasing pressure forces particles closer together, favoring the condensed states (solid and liquid). Decreasing pressure allows particles to spread out, favoring the gaseous state.
Phase Diagrams: Visualizing State Transitions
Phase diagrams graphically represent the relationship between temperature, pressure, and the state of matter for a substance. They show the conditions under which a substance exists as a solid, liquid, or gas, as well as the boundaries between these phases (melting point, boiling point, etc.).
Beyond the Three Basic States: Plasma and Bose-Einstein Condensates
While solids, liquids, and gases constitute the most common states of matter, other states exist under extreme conditions:
Plasma: The Ionized State
Plasma is a highly ionized gas, characterized by the presence of free electrons and ions. This state is common in stars, lightning, and fluorescent lights.
Bose-Einstein Condensates: The Supercooled State
Bose-Einstein condensates (BECs) are formed at extremely low temperatures and represent a state where a large number of atoms occupy the lowest quantum state. They exhibit macroscopic quantum phenomena.
Applications of State Knowledge
Understanding the states of matter and their relationships to the periodic table has numerous applications:
-
Material Science: Designing materials with specific properties (strength, conductivity, malleability) requires knowledge of the interatomic and intermolecular forces responsible for their states.
-
Chemical Engineering: Optimizing chemical processes, such as distillation and crystallization, requires understanding phase transitions.
-
Environmental Science: Studying atmospheric processes and climate change involves understanding the behavior of gases and their interactions.
-
Medicine: Many pharmaceuticals exist in various solid and liquid forms, and understanding their properties is crucial for formulation and delivery.
Conclusion: A Dynamic Periodic Table
The periodic table is more than just a static arrangement of elements. It provides a framework for understanding the diverse properties of elements, including their behavior in different states of matter. By considering factors like atomic mass, electronegativity, and intermolecular forces, we can better comprehend the relationships between the periodic table and the dynamic world of solids, liquids, and gases. Further exploration into phase transitions, extreme states of matter, and the applications of this knowledge will continue to expand our understanding of the universe and its constituent materials.
Latest Posts
Latest Posts
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
-
What Are The Functions Of The Family
Mar 18, 2025
-
Trends In The Periodic Table Melting Point
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table Solid Liquid And Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
