Wo Letter Symbol From The Periodic Table.
Muz Play
Mar 17, 2025 · 14 min read
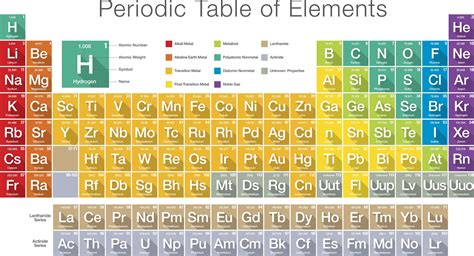
Table of Contents
Two-Letter Symbols in the Periodic Table: A Deep Dive
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number, electron configuration, and recurring chemical properties. While most elements boast a single-letter or two-letter symbol, understanding the significance of these symbols, especially the two-letter ones, offers a deeper appreciation of the table's structure and the elements themselves. This comprehensive guide delves into the fascinating world of two-letter element symbols, exploring their origins, the elements they represent, and their unique characteristics.
The Significance of Element Symbols
Element symbols are shorthand notations that represent the elements on the periodic table. These symbols, typically one or two letters derived from the element's name (often its Latin name), are internationally recognized and crucial for scientific communication and chemical equations. The consistent use of symbols transcends language barriers, enabling global collaboration in scientific research.
The use of two-letter symbols primarily arises from the necessity to differentiate elements with similar single-letter abbreviations. For instance, if only single-letter symbols were used, both carbon (C) and calcium (Ca) could potentially be represented by the letter "C," leading to ambiguity and potential errors in chemical formulas and equations. The two-letter system resolves this ambiguity, ensuring clarity and precision.
Two-Letter Symbols: A Closer Look
Let's delve into a detailed analysis of each element represented by a two-letter symbol:
He: Helium
- Atomic Number: 2
- Group: 18 (Noble Gases)
- Period: 1
- Characteristics: Helium is an inert, colorless, odorless, tasteless, non-toxic, and monatomic gas. It's the second lightest element and is known for its extremely low boiling point, making it useful for cryogenics and applications requiring low temperatures. Its low reactivity makes it safe to use in balloons and other applications where a non-reactive gas is needed. Helium is also crucial in MRI machines, providing a supercooled environment for the superconducting magnets.
Li: Lithium
- Atomic Number: 3
- Group: 1 (Alkali Metals)
- Period: 2
- Characteristics: Lithium is a soft, silvery-white alkali metal. It's the lightest metal and is highly reactive, especially with water. Despite its reactivity, lithium compounds find numerous applications, including in batteries (lithium-ion batteries are ubiquitous in portable electronics), ceramics, and certain alloys. Lithium also plays a significant role in treating bipolar disorder.
Be: Beryllium
- Atomic Number: 4
- Group: 2 (Alkaline Earth Metals)
- Period: 2
- Characteristics: Beryllium is a strong, lightweight metal with high thermal conductivity and high melting point. While it boasts desirable physical properties, beryllium is toxic and is handled with stringent safety protocols. It finds applications in aerospace, nuclear reactors, and as an alloying agent in various materials, primarily where its lightweight and high strength are crucial. However, its toxicity limits widespread use.
Ne: Neon
- Atomic Number: 10
- Group: 18 (Noble Gases)
- Period: 2
- Characteristics: Neon is a noble gas, like helium, known for its distinctive red-orange glow when energized electrically. This property makes neon a popular choice for advertising signs. Its chemical inertness ensures it doesn't react readily with other elements, making it safe for these applications. Neon's unique spectral lines also play a role in various scientific instruments.
Na: Sodium
- Atomic Number: 11
- Group: 1 (Alkali Metals)
- Period: 3
- Characteristics: Sodium is a soft, silvery-white, highly reactive alkali metal that readily reacts with water, producing hydrogen gas and heat. It's crucial in biological systems, playing a significant role in nerve impulse transmission and fluid balance. Sodium chloride (common table salt) is a crucial sodium compound found extensively in daily life. Sodium compounds also find use in various industrial applications.
Mg: Magnesium
- Atomic Number: 12
- Group: 2 (Alkaline Earth Metals)
- Period: 3
- Characteristics: Magnesium is a lightweight, strong metal that is crucial for various biological processes and is widely used in manufacturing. It is used as a structural material in various alloys for its strength-to-weight ratio and is also crucial in photographic flash bulbs due to its ability to burn brightly when ignited. In biology, magnesium ions play a vital role in enzymatic reactions and are necessary for proper cellular function.
Al: Aluminum
- Atomic Number: 13
- Group: 13 (Boron Group)
- Period: 3
- Characteristics: Aluminum is a lightweight, ductile, and corrosion-resistant metal. Its abundance in the Earth's crust and its recyclability have made it an essential material in countless applications, from packaging and transportation to construction and electronics. The use of aluminum has revolutionized many industries due to its versatility and cost-effectiveness.
Si: Silicon
- Atomic Number: 14
- Group: 14 (Carbon Group)
- Period: 3
- Characteristics: Silicon is a metalloid, meaning it exhibits properties of both metals and non-metals. It's a crucial component of semiconductors, forming the basis of modern electronics, including microchips and integrated circuits. Silicon is also a major component of many industrial materials such as glass, concrete, and silicones.
P: Phosphorus
- Atomic Number: 15
- Group: 15 (Pnictogens)
- Period: 3
- Characteristics: Phosphorus is a highly reactive non-metal that exists in several allotropic forms, each exhibiting different properties. White phosphorus is highly toxic and flammable, while red phosphorus is relatively stable. Phosphorus is a crucial element in biological systems, playing a critical role in DNA, RNA, and ATP (adenosine triphosphate), the energy currency of cells. It's also found in fertilizers and various industrial chemicals.
S: Sulfur
- Atomic Number: 16
- Group: 16 (Chalcogens)
- Period: 3
- Characteristics: Sulfur is a non-metal known for its yellow crystalline form. It's a crucial element in various biological processes, particularly in the formation of proteins. Sulfur is also found in many industrial applications, including the production of sulfuric acid, a vital chemical in numerous industries. Sulfur compounds contribute to various environmental issues like acid rain.
Cl: Chlorine
- Atomic Number: 17
- Group: 17 (Halogens)
- Period: 3
- Characteristics: Chlorine is a highly reactive halogen gas, pale green in color, with a pungent odor. In its elemental form, it's toxic, but it's crucial in various applications when it is part of compounds. Chlorine is used extensively in water purification as a disinfectant, killing harmful bacteria and viruses. It also plays a role in the production of various chemicals and polymers.
Ar: Argon
- Atomic Number: 18
- Group: 18 (Noble Gases)
- Period: 3
- Characteristics: Argon is a noble gas known for its inertness and its use in various applications where a non-reactive atmosphere is required. It's utilized in welding to protect the weld from oxidation, in lighting applications, and in the semiconductor industry. Argon's inertness makes it suitable for preserving delicate samples and preventing unwanted chemical reactions.
K: Potassium
- Atomic Number: 19
- Group: 1 (Alkali Metals)
- Period: 4
- Characteristics: Potassium is a soft, silvery-white, highly reactive alkali metal. Like sodium, it plays a crucial role in biological systems, regulating nerve impulses and muscle contractions. Potassium deficiency can lead to various health issues. It's also important in various industrial applications.
Ca: Calcium
- Atomic Number: 20
- Group: 2 (Alkaline Earth Metals)
- Period: 4
- Characteristics: Calcium is an alkaline earth metal crucial for various biological processes, including bone formation and muscle contraction. It's the fifth most abundant element in the human body. Calcium compounds are widely used in various industrial applications, including construction materials (cement) and the production of various chemicals.
Sc: Scandium
- Atomic Number: 21
- Group: 3 (Scandium Group)
- Period: 4
- Characteristics: Scandium is a transition metal, relatively rare in the Earth's crust, with applications in high-intensity lighting and certain alloys. While less common than other transition metals, it's finding increasing uses in specialized applications.
Ti: Titanium
- Atomic Number: 22
- Group: 4 (Titanium Group)
- Period: 4
- Characteristics: Titanium is a strong, lightweight, corrosion-resistant transition metal highly valued for its properties. It's used extensively in aerospace, biomedical implants, and sporting goods. Titanium's resistance to corrosion makes it ideal for applications in harsh environments.
V: Vanadium
- Atomic Number: 23
- Group: 5 (Vanadium Group)
- Period: 4
- Characteristics: Vanadium is a transition metal primarily used in alloys to improve strength and corrosion resistance. It's also used in some catalysts and in nuclear reactors.
Cr: Chromium
- Atomic Number: 24
- Group: 6 (Chromium Group)
- Period: 4
- Characteristics: Chromium is a hard, brittle, lustrous transition metal known for its high corrosion resistance. This property makes it valuable for plating other metals to protect them from corrosion and for applications in stainless steel. Chromium compounds are also used in various industrial applications.
Mn: Manganese
- Atomic Number: 25
- Group: 7 (Manganese Group)
- Period: 4
- Characteristics: Manganese is a brittle, grayish-white transition metal primarily used in steel production to improve its strength and hardness. Manganese compounds also play a role in various industrial processes.
Fe: Iron
- Atomic Number: 26
- Group: 8 (Iron Group)
- Period: 4
- Characteristics: Iron is a common and essential transition metal found extensively in nature. It’s a crucial component of steel, one of the most important structural materials used worldwide. Iron plays an essential role in various biological processes, including oxygen transport in blood (hemoglobin).
Co: Cobalt
- Atomic Number: 27
- Group: 9 (Cobalt Group)
- Period: 4
- Characteristics: Cobalt is a hard, brittle, lustrous transition metal often used in alloys to enhance their hardness and corrosion resistance. It's also crucial in certain catalysts and in the production of magnets. Cobalt compounds are used in various industrial applications.
Ni: Nickel
- Atomic Number: 28
- Group: 10 (Nickel Group)
- Period: 4
- Characteristics: Nickel is a hard, silvery-white transition metal widely used in various alloys, particularly stainless steel. It's also used in nickel-cadmium batteries and various catalysts.
Cu: Copper
- Atomic Number: 29
- Group: 11 (Copper Group)
- Period: 4
- Characteristics: Copper is a reddish-brown transition metal known for its excellent electrical conductivity. It's extensively used in electrical wiring and various other applications where its conductive properties are crucial.
Zn: Zinc
- Atomic Number: 30
- Group: 12 (Zinc Group)
- Period: 4
- Characteristics: Zinc is a bluish-white transition metal used widely in various alloys to improve their corrosion resistance and strength. Zinc is also essential in many biological processes.
Ga: Gallium
- Atomic Number: 31
- Group: 13 (Boron Group)
- Period: 4
- Characteristics: Gallium is a soft, silvery metal with a low melting point and unique properties. It's used in semiconductors and various other applications.
Ge: Germanium
- Atomic Number: 32
- Group: 14 (Carbon Group)
- Period: 4
- Characteristics: Germanium is a metalloid with semiconductor properties. It was historically important in transistors but has been largely replaced by silicon in many applications.
As: Arsenic
- Atomic Number: 33
- Group: 15 (Pnictogens)
- Period: 4
- Characteristics: Arsenic is a metalloid with toxic properties. It finds limited use in some alloys and semiconductors, but its toxicity restricts its applications.
Se: Selenium
- Atomic Number: 34
- Group: 16 (Chalcogens)
- Period: 4
- Characteristics: Selenium is a metalloid with semiconductor properties. It's used in photocells and as a nutritional supplement.
Br: Bromine
- Atomic Number: 35
- Group: 17 (Halogens)
- Period: 4
- Characteristics: Bromine is a reddish-brown liquid halogen, one of the few elements that are liquid at room temperature. It's used in various chemical compounds.
Kr: Krypton
- Atomic Number: 36
- Group: 18 (Noble Gases)
- Period: 4
- Characteristics: Krypton is a noble gas used in some lighting applications.
Rb: Rubidium
- Atomic Number: 37
- Group: 1 (Alkali Metals)
- Period: 5
- Characteristics: Rubidium is a highly reactive alkali metal with applications in some specialized areas.
Sr: Strontium
- Atomic Number: 38
- Group: 2 (Alkaline Earth Metals)
- Period: 5
- Characteristics: Strontium is an alkaline earth metal with applications in fireworks and some specialized materials.
Y: Yttrium
- Atomic Number: 39
- Group: 3 (Scandium Group)
- Period: 5
- Characteristics: Yttrium is a transition metal used in various alloys and in high-temperature superconductors.
Zr: Zirconium
- Atomic Number: 40
- Group: 4 (Titanium Group)
- Period: 5
- Characteristics: Zirconium is a transition metal with high corrosion resistance, used in nuclear reactors and other specialized applications.
Nb: Niobium
- Atomic Number: 41
- Group: 5 (Vanadium Group)
- Period: 5
- Characteristics: Niobium is a transition metal used in various alloys to improve strength and corrosion resistance.
Mo: Molybdenum
- Atomic Number: 42
- Group: 6 (Chromium Group)
- Period: 5
- Characteristics: Molybdenum is a transition metal used in steel alloys to enhance their strength and high-temperature performance. It's also used in catalysts.
Tc: Technetium
- Atomic Number: 43
- Group: 7 (Manganese Group)
- Period: 5
- Characteristics: Technetium is a radioactive transition metal with applications in nuclear medicine.
Ru: Ruthenium
- Atomic Number: 44
- Group: 8 (Iron Group)
- Period: 5
- Characteristics: Ruthenium is a transition metal used in some alloys and catalysts.
Rh: Rhodium
- Atomic Number: 45
- Group: 9 (Cobalt Group)
- Period: 5
- Characteristics: Rhodium is a transition metal used in catalysts and jewelry.
Pd: Palladium
- Atomic Number: 46
- Group: 10 (Nickel Group)
- Period: 5
- Characteristics: Palladium is a transition metal used in catalysts and jewelry.
Ag: Silver
- Atomic Number: 47
- Group: 11 (Copper Group)
- Period: 5
- Characteristics: Silver is a transition metal known for its high electrical conductivity and use in jewelry, photography, and electronics.
Cd: Cadmium
- Atomic Number: 48
- Group: 12 (Zinc Group)
- Period: 5
- Characteristics: Cadmium is a transition metal used in some alloys and batteries, but its toxicity limits its use.
In: Indium
- Atomic Number: 49
- Group: 13 (Boron Group)
- Period: 5
- Characteristics: Indium is a soft, silvery metal used in some alloys and semiconductors.
Sn: Tin
- Atomic Number: 50
- Group: 14 (Carbon Group)
- Period: 5
- Characteristics: Tin is a silvery-white metal used in alloys, coatings, and various other applications.
Sb: Antimony
- Atomic Number: 51
- Group: 15 (Pnictogens)
- Period: 5
- Characteristics: Antimony is a metalloid used in alloys and some specialized applications.
Te: Tellurium
- Atomic Number: 52
- Group: 16 (Chalcogens)
- Period: 5
- Characteristics: Tellurium is a metalloid used in some alloys and semiconductors.
I: Iodine
- Atomic Number: 53
- Group: 17 (Halogens)
- Period: 5
- Characteristics: Iodine is a dark gray, crystalline halogen essential for thyroid hormone production.
Xe: Xenon
- Atomic Number: 54
- Group: 18 (Noble Gases)
- Period: 5
- Characteristics: Xenon is a noble gas used in some lighting applications.
Cs: Cesium
- Atomic Number: 55
- Group: 1 (Alkali Metals)
- Period: 6
- Characteristics: Cesium is a highly reactive alkali metal with applications in atomic clocks and some specialized areas.
Ba: Barium
- Atomic Number: 56
- Group: 2 (Alkaline Earth Metals)
- Period: 6
- Characteristics: Barium is an alkaline earth metal used in some alloys and in fireworks.
La: Lanthanum
- Atomic Number: 57
- Group: 3 (Scandium Group)
- Period: 6
- Characteristics: Lanthanum is a lanthanide used in some alloys and catalysts.
Hf: Hafnium
- Atomic Number: 72
- Group: 4 (Titanium Group)
- Period: 6
- Characteristics: Hafnium is a transition metal used in some alloys and in nuclear reactors.
Ta: Tantalum
- Atomic Number: 73
- Group: 5 (Vanadium Group)
- Period: 6
- Characteristics: Tantalum is a transition metal used in electronics and some specialized applications.
W: Tungsten
- Atomic Number: 74
- Group: 6 (Chromium Group)
- Period: 6
- Characteristics: Tungsten is a transition metal known for its high melting point and use in light bulb filaments and high-temperature applications.
Re: Rhenium
- Atomic Number: 75
- Group: 7 (Manganese Group)
- Period: 6
- Characteristics: Rhenium is a transition metal used in some alloys and catalysts.
Os: Osmium
- Atomic Number: 76
- Group: 8 (Iron Group)
- Period: 6
- Characteristics: Osmium is a transition metal known for its high density and use in some specialized applications.
Ir: Iridium
- Atomic Number: 77
- Group: 9 (Cobalt Group)
- Period: 6
- Characteristics: Iridium is a transition metal used in some alloys and catalysts. It is known for its high corrosion resistance.
Pt: Platinum
- Atomic Number: 78
- Group: 10 (Nickel Group)
- Period: 6
- Characteristics: Platinum is a transition metal known for its high corrosion resistance, use in catalysts, and jewelry.
Au: Gold
- Atomic Number: 79
- Group: 11 (Copper Group)
- Period: 6
- Characteristics: Gold is a transition metal known for its high malleability, ductility, and use in jewelry, electronics, and finance.
Hg: Mercury
- Atomic Number: 80
- Group: 12 (Zinc Group)
- Period: 6
- Characteristics: Mercury is a liquid transition metal at room temperature, known for its toxicity and use in some specialized applications.
Tl: Thallium
- Atomic Number: 81
- Group: 13 (Boron Group)
- Period: 6
- Characteristics: Thallium is a heavy metal with toxic properties, limiting its uses.
Pb: Lead
- Atomic Number: 82
- Group: 14 (Carbon Group)
- Period: 6
- Characteristics: Lead is a heavy metal with toxic properties, limiting its uses, although it was historically widely used.
Bi: Bismuth
- Atomic Number: 83
- Group: 15 (Pnictogens)
- Period: 6
- Characteristics: Bismuth is a heavy metal with low toxicity compared to other heavy metals. It's used in some alloys and pharmaceuticals.
Po: Polonium
- Atomic Number: 84
- Group: 16 (Chalcogens)
- Period: 6
- Characteristics: Polonium is a highly radioactive element with limited uses.
At: Astatine
- Atomic Number: 85
- Group: 17 (Halogens)
- Period: 6
- Characteristics: Astatine is a highly radioactive element with very limited uses and research.
Rn: Radon
- Atomic Number: 86
- Group: 18 (Noble Gases)
- Period: 6
- Characteristics: Radon is a radioactive noble gas, a significant health hazard due to its radioactivity.
This comprehensive overview of the two-letter elements in the periodic table highlights their importance in various fields, from industrial applications to biological processes and medical treatments. The consistent use of these two-letter symbols across the scientific community ensures clear and precise communication and collaboration, facilitating advancements in research and development. Understanding the nuances of these symbols and the elements they represent provides a deeper understanding of the periodic table and its profound implications for our world.
Latest Posts
Latest Posts
-
Which One Leaves The Solution Untouched
Mar 18, 2025
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Wo Letter Symbol From The Periodic Table. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
