Are Ionic Compounds Made Of Metals And Nonmetals
Muz Play
Mar 16, 2025 · 6 min read
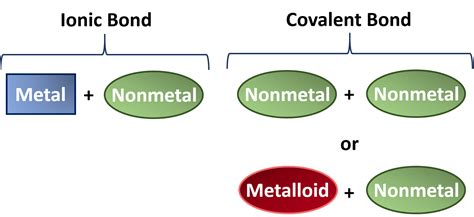
Table of Contents
Are Ionic Compounds Made of Metals and Nonmetals? A Deep Dive into Ionic Bonding
Ionic compounds are ubiquitous in our world, forming the basis of countless materials we interact with daily, from the salt we use to season our food to the minerals that make up our planet's crust. Understanding their fundamental nature – the combination of metals and nonmetals – is crucial to grasping their unique properties and applications. This article delves into the intricacies of ionic bonding, exploring the characteristics of metals and nonmetals, the formation of ionic compounds, and the exceptions that prove the rule.
The Dance of Electrons: Understanding Metallic and Nonmetallic Behavior
Before diving into the formation of ionic compounds, let's establish a firm understanding of the properties that define metals and nonmetals. These properties are fundamentally linked to their electron configurations and how readily they gain or lose electrons.
Metals: Electron Donors
Metals, located on the left side of the periodic table, are characterized by their tendency to lose electrons. This is due to their relatively low electronegativity – a measure of an atom's ability to attract electrons in a chemical bond. They have loosely held valence electrons in their outermost electron shell. Losing these electrons results in a stable, positively charged ion, called a cation. This electron loss is what allows metals to conduct electricity and heat effectively; the mobile electrons are free to move throughout the metallic lattice.
Key Characteristics of Metals:
- Low electronegativity: They readily lose electrons.
- Good electrical and thermal conductivity: Due to mobile electrons.
- Malleable and ductile: Their atoms can easily slide past one another.
- Luster: They reflect light well.
- Generally solid at room temperature (except for mercury).
Nonmetals: Electron Acceptors
Nonmetals, positioned on the right side of the periodic table, exhibit the opposite behavior. They have a high electronegativity, meaning they strongly attract electrons. They tend to gain electrons to achieve a stable electron configuration, usually a full outermost shell. This gain of electrons results in a negatively charged ion, called an anion.
Key Characteristics of Nonmetals:
- High electronegativity: They readily gain electrons.
- Poor electrical and thermal conductivity (except for graphite): Electrons are tightly held.
- Brittle: Their structures are not easily deformed.
- Dull appearance (lack of luster): They don't reflect light as effectively as metals.
- Can exist in all three states of matter at room temperature.
The Formation of Ionic Compounds: An Electrostatic Attraction
Ionic compounds are formed through the electrostatic attraction between positively charged cations (metal ions) and negatively charged anions (nonmetal ions). This attraction arises from the significant difference in electronegativity between metals and nonmetals. The metal atom, eager to lose electrons, transfers one or more electrons to the nonmetal atom, which readily accepts them. This transfer of electrons creates ions with opposite charges, leading to a strong electrostatic force that holds them together in a crystalline lattice structure.
The Process:
- Electron Transfer: A metal atom loses one or more electrons to become a positively charged cation.
- Electron Gain: A nonmetal atom gains one or more electrons to become a negatively charged anion.
- Electrostatic Attraction: The oppositely charged ions attract each other, forming an ionic bond.
- Crystal Lattice Formation: The ions arrange themselves in a regular, repeating three-dimensional structure called a crystal lattice, maximizing electrostatic attractions and minimizing repulsions.
This process is driven by the inherent instability of individual metal and nonmetal atoms in their neutral states. By forming ions and achieving stable electron configurations (often resembling noble gases), they significantly lower their overall energy, leading to a more stable system.
Examples of Ionic Compounds: A Diverse World of Substances
Numerous examples showcase the widespread existence of ionic compounds formed from metals and nonmetals. These include:
- Sodium Chloride (NaCl): Common table salt, formed from the sodium cation (Na⁺) and the chloride anion (Cl⁻).
- Potassium Iodide (KI): Used in dietary supplements and as a disinfectant, formed from potassium cation (K⁺) and iodide anion (I⁻).
- Magnesium Oxide (MgO): Used in refractory materials and as a food additive, formed from magnesium cation (Mg²⁺) and oxide anion (O²⁻).
- Calcium Carbonate (CaCO₃): A major component of limestone and marble, formed from calcium cation (Ca²⁺) and carbonate anion (CO₃²⁻).
- Aluminum Oxide (Al₂O₃): Found in gems like rubies and sapphires, formed from aluminum cation (Al³⁺) and oxide anion (O²⁻).
Properties of Ionic Compounds: A Consequence of Ionic Bonding
The strong electrostatic forces within the ionic lattice dictate the unique properties of ionic compounds:
- High melting and boiling points: The strong ionic bonds require significant energy to overcome.
- Crystalline structure: The regular arrangement of ions in the lattice results in a crystalline solid.
- Hardness and brittleness: While strong, ionic crystals are brittle because the displacement of ions can lead to repulsion between like charges.
- Solubility in polar solvents: Ionic compounds often dissolve in polar solvents like water because the polar molecules can interact with the charged ions.
- Electrical conductivity when molten or dissolved: The ions become mobile and can conduct electricity.
Exceptions to the Rule: Polyatomic Ions and Covalent Character
While the general rule states that ionic compounds are formed from metals and nonmetals, there are some exceptions and nuances to consider:
Polyatomic Ions: Groups of Atoms with a Charge
Polyatomic ions are groups of atoms that carry an overall charge. These ions can participate in ionic bonding, even though they contain both metal and nonmetal atoms within their structure. Examples include the nitrate ion (NO₃⁻), sulfate ion (SO₄²⁻), and ammonium ion (NH₄⁺). These ions act as single units in forming ionic compounds, such as ammonium chloride (NH₄Cl) and potassium nitrate (KNO₃).
Covalent Character in Ionic Bonds: A Spectrum of Bonding
The concept of electronegativity helps explain that ionic bonding isn't always a clear-cut transfer of electrons. Instead, it exists on a spectrum. The greater the difference in electronegativity between two atoms, the more ionic the bond becomes. However, even in compounds considered predominantly ionic, there's often some degree of covalent character—meaning there's some sharing of electrons in addition to the transfer. This is especially true when the electronegativity difference is not extremely large.
Transition Metal Compounds: A Complexity of Bonding
Transition metals, located in the d-block of the periodic table, often exhibit variable oxidation states. This means they can lose different numbers of electrons to form ions with varying charges. This results in a greater complexity in the bonding in transition metal compounds, with some exhibiting a significant degree of covalent character. Furthermore, the presence of d-electrons can lead to the formation of complex ions and coordination compounds, which blur the lines between purely ionic and covalent bonding.
Conclusion: A Foundation of Chemistry and Material Science
The formation of ionic compounds from metals and nonmetals is a cornerstone of chemistry. Understanding the principles of ionic bonding, the properties of metals and nonmetals, and the exceptions to the general rule provides a strong foundation for comprehending the vast array of materials that constitute our world. From the simplest salts to complex minerals and technologically advanced materials, ionic compounds play a vital role in our daily lives and scientific endeavors. Continuing research in this area continues to refine our understanding of the subtleties of chemical bonding and its impact on material properties. The seemingly simple interaction between metals and nonmetals leads to a rich diversity of compounds and a fascinating study in chemical interactions.
Latest Posts
Latest Posts
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Are Ionic Compounds Made Of Metals And Nonmetals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
