Are Lipids Polar Or Non Polar
Muz Play
Mar 23, 2025 · 6 min read
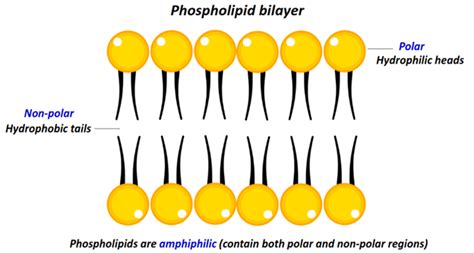
Table of Contents
Are Lipids Polar or Nonpolar? Understanding the Chemistry of Fats
Lipids are a diverse group of biological molecules defined by their insolubility in water. This characteristic stems from their predominantly nonpolar nature, a crucial factor in their diverse functions within living organisms. While the blanket statement "lipids are nonpolar" holds true in most cases, a nuanced understanding reveals exceptions and variations within this broad class of molecules. This article will delve into the polarity of lipids, exploring the underlying chemistry, the exceptions to the rule, and the implications of their hydrophobic nature for their biological roles.
The Chemistry of Polarity: A Quick Refresher
Before diving into the specifics of lipids, let's briefly review the concept of polarity. Polarity refers to the uneven distribution of electrical charge within a molecule. This arises from differences in electronegativity between atoms. Electronegativity is the tendency of an atom to attract electrons in a chemical bond. When atoms with significantly different electronegativities bond, the electrons are pulled more towards the more electronegative atom, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom. This creates a dipole moment, resulting in a polar molecule.
Water (H₂O) is a classic example of a polar molecule. Oxygen is significantly more electronegative than hydrogen, creating a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. This polarity allows water molecules to form strong hydrogen bonds with each other and with other polar molecules.
Nonpolar molecules, on the other hand, have an even distribution of charge. This usually occurs when atoms with similar electronegativities bond, or when the molecule's geometry cancels out any dipole moments. Nonpolar molecules generally interact weakly with water, preferring to interact with other nonpolar molecules—a phenomenon known as hydrophobic interaction.
The Predominantly Nonpolar Nature of Lipids
The majority of lipids are composed primarily of long hydrocarbon chains, which are characterized by carbon-carbon and carbon-hydrogen bonds. Carbon and hydrogen have very similar electronegativities, making these bonds essentially nonpolar. The long, nonpolar hydrocarbon chains are the primary reason why most lipids are insoluble in water and exhibit hydrophobic behavior.
Examples of Nonpolar Lipids:
-
Triglycerides: These are the most common type of lipid, composed of three fatty acids esterified to a glycerol molecule. Fatty acids are long hydrocarbon chains with a carboxyl group (-COOH) at one end. While the carboxyl group is polar, the long hydrocarbon tail dominates, rendering the entire triglyceride molecule largely nonpolar.
-
Fatty Acids: As mentioned, fatty acids are building blocks of many lipids. The long hydrocarbon chain makes them hydrophobic, and even though they have a polar carboxyl group at one end, this region is relatively small compared to the nonpolar tail.
-
Cholesterol: This lipid is a crucial component of cell membranes. It contains a steroid nucleus, which is a complex ring structure, and a short hydrocarbon tail. While it has some polar hydroxyl (-OH) groups, the bulk of the molecule is nonpolar.
Exceptions: Polar Lipids and Amphipathic Lipids
While the majority of lipids are nonpolar, some exceptions exist. These exceptions often have significant biological implications.
Polar Lipids: These lipids contain a significant proportion of polar functional groups, affecting their overall polarity. The presence of these polar groups leads to increased solubility in water compared to the predominantly nonpolar lipids.
Examples of Polar Lipids:
-
Phospholipids: These are major components of cell membranes. They consist of a glycerol backbone, two fatty acids, a phosphate group, and a polar head group. The phosphate group and the polar head group are highly polar, while the fatty acid tails remain nonpolar. This dual nature makes phospholipids amphipathic, meaning they possess both polar and nonpolar regions.
-
Sphingolipids: Similar to phospholipids, sphingolipids are also components of cell membranes and contain a polar head group and nonpolar tail. They differ in their backbone structure compared to phospholipids.
Amphipathic Lipids: As highlighted in the phospholipid example, many lipids exhibit amphipathic character. This dual nature is essential for their function in biological membranes. The polar head groups interact with the aqueous environment (cytoplasm and extracellular fluid), while the nonpolar tails cluster together, forming a hydrophobic core within the membrane. This arrangement creates a selectively permeable barrier that regulates the passage of substances into and out of cells.
The Importance of Lipid Hydrophobicity
The hydrophobic nature of lipids is crucial for their various biological roles:
-
Membrane Structure: The hydrophobic interaction between lipid tails is the driving force behind the formation of biological membranes. These membranes create compartments within cells and regulate the movement of molecules across cell boundaries.
-
Energy Storage: Triglycerides store energy efficiently. Their hydrophobic nature allows them to be packed densely in adipose tissue without attracting water molecules, minimizing weight and maximizing energy storage.
-
Hormone Production: Steroid hormones, derived from cholesterol, are important signaling molecules. The nonpolar nature of steroids allows them to easily cross cell membranes and interact with intracellular receptors.
-
Insulation: Lipids provide insulation, protecting organisms from extreme temperatures. Adipose tissue, rich in triglycerides, acts as an insulator, preventing heat loss.
-
Protection of Vital Organs: Fat pads around vital organs cushion them from impact.
Addressing Common Misconceptions
It's important to clarify some common misunderstandings surrounding lipid polarity:
-
Not all lipids are equally nonpolar: The degree of nonpolarity varies significantly among different lipids, depending on their chemical structure and the proportion of polar functional groups present.
-
The presence of even one polar group can significantly alter behavior: While a large hydrocarbon chain dominates, a strategically placed polar group (as in phospholipids) can drastically alter the lipid's interaction with water and other molecules.
-
The term "nonpolar" is relative: While most lipids are considered nonpolar in the context of their overall interaction with water, they still exhibit interactions with other molecules based on their specific functional groups.
Conclusion: A Diverse Class of Molecules
In summary, while the general statement that lipids are nonpolar is largely accurate, it's crucial to acknowledge the significant diversity within this class of biomolecules. The polarity of lipids greatly influences their solubility, interactions with other molecules, and consequently their biological functions. From the predominantly nonpolar triglycerides responsible for energy storage to the amphipathic phospholipids that form the basis of cell membranes, the diversity in lipid structure reflects the multitude of vital roles they play in living organisms. Understanding this diversity is fundamental to appreciating the complexity and sophistication of biological systems. Future research continues to unveil the intricacies of lipid interactions and their implications for health and disease. The ongoing investigation into lipidomics promises even deeper insights into the dynamic world of these essential biomolecules.
Latest Posts
Latest Posts
-
Is Water A Homogeneous Or Heterogeneous
Mar 25, 2025
-
What Is A Negatively Charged Ion Called
Mar 25, 2025
-
Changing The Ph Can Cause A Protein To
Mar 25, 2025
-
How Is Global Stratification Different From Social Stratification
Mar 25, 2025
-
Integration Of Even And Odd Functions
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Are Lipids Polar Or Non Polar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
