__________ Are The Most Organized State Of Matter. Responses
Muz Play
Mar 25, 2025 · 6 min read
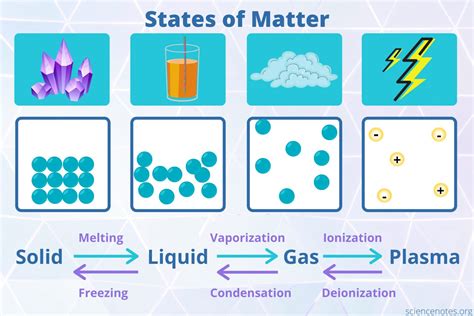
Table of Contents
Solids: The Most Organized State of Matter
Solids are the most organized state of matter. This statement isn't just a simple fact; it's the foundation upon which we understand the behavior of materials and the intricate dance of atoms and molecules. Understanding the organization within solids unlocks the secrets to their unique properties and explains why they behave so differently from liquids and gases. This article will delve deep into the defining characteristics of solids, exploring their various types, the forces that govern their structure, and the fascinating implications of their remarkable organization.
The Defining Characteristics of Solids
What truly sets solids apart is their fixed shape and volume. Unlike liquids, which adapt to the shape of their container, and gases, which expand to fill any available space, solids resolutely maintain their structure. This unwavering consistency stems from the strong intermolecular forces that bind their constituent particles together. These forces, be they ionic bonds, covalent bonds, metallic bonds, or weaker intermolecular forces like van der Waals forces and hydrogen bonds, hold the particles in relatively fixed positions, creating a rigid framework.
Strong Intermolecular Forces: The Glue that Holds Solids Together
The strength of these intermolecular forces dictates many of the properties of a solid, including its hardness, melting point, and electrical conductivity. Stronger forces result in solids that are harder to deform, require higher temperatures to melt, and might exhibit unique electrical properties.
-
Ionic Solids: These solids are formed by the electrostatic attraction between positively and negatively charged ions. The strong Coulombic forces between these ions lead to high melting points and brittle nature. Examples include table salt (NaCl) and many other salts.
-
Covalent Solids: These solids are characterized by strong covalent bonds between atoms, forming a network or lattice structure. Diamond, with its tetrahedral arrangement of carbon atoms, is a prime example of a covalent solid, exhibiting exceptional hardness and high melting point. Silicon dioxide (quartz) is another example.
-
Metallic Solids: Metals are held together by metallic bonds, where electrons are delocalized and shared among a "sea" of electrons. This delocalized electron cloud allows for high electrical and thermal conductivity, malleability, and ductility.
-
Molecular Solids: These solids consist of molecules held together by weaker intermolecular forces, such as van der Waals forces and hydrogen bonds. These forces are significantly weaker than ionic, covalent, or metallic bonds, leading to lower melting points and greater softness. Ice (H₂O) is a classic example of a molecular solid.
The Different Types of Solid Structures
The organization within solids isn't just about the strength of the bonds; it's also about the arrangement of the particles. This arrangement determines the crystalline structure of the solid, which dictates many of its physical properties.
Crystalline Solids: The Orderly Arrangement
Crystalline solids are characterized by a highly ordered, three-dimensional arrangement of their constituent particles. These particles – atoms, ions, or molecules – are arranged in a repeating pattern, forming a lattice structure that extends throughout the entire solid. This long-range order is what gives crystalline solids their sharp melting points and often their distinctive geometrical shapes (crystals). Examples include table salt (cubic crystal system), quartz (hexagonal), and diamonds (cubic).
Amorphous Solids: The Lack of Long-Range Order
In contrast to crystalline solids, amorphous solids lack this long-range order. While there is some short-range order among the particles, the overall arrangement is random and disordered. This lack of organization often leads to properties different from their crystalline counterparts. Amorphous solids often exhibit a gradual softening over a range of temperatures rather than a sharp melting point. Examples include glass, rubber, and many plastics.
The Importance of Long-Range Order in Solids
The high degree of organization in crystalline solids is responsible for many of their unique and useful properties. This order dictates the way light interacts with the solid (e.g., causing birefringence in some crystals), how it conducts electricity (e.g., anisotropic conductivity in some crystals), and how it responds to stress (e.g., anisotropic mechanical properties).
Anisotropy: The Directional Dependence of Properties
The directional dependence of properties in crystalline solids, known as anisotropy, is a direct consequence of their ordered structure. For example, the speed of light can vary depending on the direction it travels through a crystal. Similarly, the mechanical strength of a crystal might be different depending on the direction of applied force. This is not observed in amorphous solids, which exhibit isotropic properties (properties that are independent of direction).
Crystal Defects: Imperfections with Significant Implications
Even in highly ordered crystalline solids, imperfections or defects exist. These defects, such as vacancies (missing atoms), interstitials (extra atoms in the lattice), and dislocations (disruptions in the lattice structure), can significantly influence the physical properties of the material. While seemingly detrimental, controlled introduction of defects can be used to enhance properties, for example, making materials stronger or more ductile.
Solid-State Physics: Unlocking the Secrets of Solids
The study of solids, known as solid-state physics, is a vast and complex field that delves into the fundamental properties and behavior of solids. Researchers use various techniques, including X-ray diffraction, electron microscopy, and spectroscopy, to understand the intricate structure and properties of solids at the atomic level. This knowledge is crucial for the development of new materials with tailored properties for a wide range of applications.
Applications of Solid-State Physics: A World of Possibilities
The principles of solid-state physics have revolutionized countless technologies. The development of transistors and integrated circuits, the foundation of modern electronics, relies heavily on our understanding of the behavior of semiconductors, a class of solids with unique electrical properties. Similarly, the design of new materials for aerospace, biomedical, and energy applications relies on our ability to control and manipulate the structure and properties of solids.
The creation of advanced materials with specific properties, such as high strength, low weight, or enhanced conductivity, relies heavily on manipulating the organization at the atomic level. This includes techniques like doping (introducing impurities), alloying (mixing different metals), and nanostructuring (creating materials with features at the nanoscale).
Comparing Solids with Liquids and Gases: A Tale of Organization
The organization of matter becomes strikingly apparent when we compare the three states of matter: solids, liquids, and gases.
Solids: The Most Organized
As we've established, solids exhibit the highest degree of organization, with particles locked in a fixed arrangement. This leads to their fixed shape and volume.
Liquids: Less Organized
Liquids have less organization than solids. While the particles are still close together, they can move around and past each other, leading to a fixed volume but a variable shape.
Gases: The Least Organized
Gases have the least organization, with particles widely spaced and moving independently. This results in both variable shape and volume.
Conclusion: The Reign of Order in the Solid State
The exceptional organization of solids is the defining characteristic that sets them apart from liquids and gases. This order, dictated by the strength and arrangement of intermolecular forces, is responsible for their diverse properties and numerous applications. From the strength of steel to the conductivity of silicon, the remarkable organization within solids continues to drive innovation and technological advancements across a wide spectrum of fields. Understanding this order is key to unlocking the potential of materials and shaping the future of technology. Further research and development in solid-state physics will undoubtedly lead to even more innovative materials and applications, solidifying the importance of solids as the most organized and perhaps the most fascinating state of matter.
Latest Posts
Latest Posts
-
Active Transport Must Function Continuously Because
Mar 28, 2025
-
The Basic Functional Unit Of The Kidney Is
Mar 28, 2025
-
Weak Development Of Support Examples In Essay
Mar 28, 2025
-
On The Weak Acid Strong Base Titration Curve
Mar 28, 2025
-
Label The Cranial Dura Septa In The Figure
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about __________ Are The Most Organized State Of Matter. Responses . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
