Arrange The Fatty Acids In Order Of Increasing Melting Point
Muz Play
Mar 26, 2025 · 5 min read
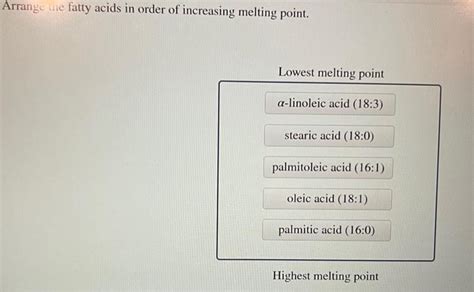
Table of Contents
Arranging Fatty Acids by Increasing Melting Point: A Comprehensive Guide
Understanding the relationship between fatty acid structure and melting point is crucial in various fields, from nutrition and food science to biochemistry and materials science. This article delves deep into the factors influencing fatty acid melting points and provides a comprehensive guide to arranging them in order of increasing melting point. We’ll explore the concepts of saturation, chain length, and cis/trans isomerism, illustrating how these structural features dictate a fatty acid's physical properties.
The Key Factors Affecting Fatty Acid Melting Point
The melting point of a fatty acid is primarily determined by three key factors:
1. Chain Length: The Longer, the Higher
Fatty acids are essentially long hydrocarbon chains with a carboxyl group (-COOH) at one end. The longer the hydrocarbon chain, the stronger the van der Waals forces between adjacent molecules. These forces require more energy to overcome, resulting in a higher melting point. Shorter chains have weaker intermolecular forces and thus lower melting points. This is a fundamental principle: longer fatty acid chains have higher melting points.
2. Degree of Saturation: Saturated vs. Unsaturated
The presence or absence of double bonds significantly impacts melting point.
-
Saturated Fatty Acids: These contain only single bonds between carbon atoms in their hydrocarbon chains. The molecules can pack tightly together, maximizing van der Waals interactions. This tight packing leads to stronger intermolecular forces and higher melting points. Think of it like neatly stacked logs – they're stable and require more energy to separate.
-
Unsaturated Fatty Acids: These contain one or more double bonds within their hydrocarbon chains. The presence of double bonds introduces kinks or bends in the molecule's structure, preventing them from packing as tightly as saturated fatty acids. This looser packing leads to weaker intermolecular forces and consequently lower melting points. Imagine trying to stack bent straws – it's much less efficient and stable.
- Monounsaturated Fatty Acids: Contain one double bond.
- Polyunsaturated Fatty Acids: Contain two or more double bonds. The more double bonds present, the lower the melting point.
3. Cis/Trans Isomerism: The Shape Matters
Unsaturated fatty acids can exist as cis or trans isomers, depending on the spatial arrangement of the atoms around the double bond.
-
Cis Isomers: In cis isomers, the hydrogen atoms attached to the carbons of the double bond are on the same side of the molecule. This creates a significant bend or kink in the chain, further hindering tight packing and leading to lower melting points compared to trans isomers. The cis configuration is much more common in naturally occurring fatty acids.
-
Trans Isomers: In trans isomers, the hydrogen atoms are on opposite sides of the double bond. This results in a more linear structure, allowing for slightly better packing than cis isomers. Consequently, trans isomers have higher melting points than their corresponding cis isomers, but still lower than saturated fatty acids.
Arranging Fatty Acids: A Practical Approach
To arrange fatty acids in order of increasing melting point, consider the three factors discussed above in a hierarchical manner:
-
Chain Length: Longer chains always have higher melting points than shorter chains, irrespective of saturation or isomerism.
-
Degree of Saturation: Saturated fatty acids always have higher melting points than unsaturated fatty acids of the same chain length. Among unsaturated fatty acids, the more double bonds present, the lower the melting point.
-
Cis/Trans Isomerism: For unsaturated fatty acids with the same chain length and number of double bonds, cis isomers have lower melting points than trans isomers.
Let's illustrate this with some examples:
Example 1: Compare butyric acid (4 carbons, saturated), palmitic acid (16 carbons, saturated), and stearic acid (18 carbons, saturated).
The order of increasing melting point would be: butyric acid < palmitic acid < stearic acid. This is solely due to the increasing chain length.
Example 2: Compare palmitic acid (16:0, saturated), oleic acid (18:1 cis), and linoleic acid (18:2 cis).
The order would be: linoleic acid < oleic acid < palmitic acid. Palmitic acid is saturated, while oleic and linoleic acids are unsaturated. Linoleic acid has two double bonds, leading to a lower melting point than oleic acid with only one double bond.
Example 3: Compare oleic acid (18:1 cis) and elaidic acid (18:1 trans).
The order would be: oleic acid < elaidic acid. Both have the same chain length and number of double bonds, but the cis configuration of oleic acid results in a lower melting point than the trans configuration of elaidic acid.
Advanced Considerations and Exceptions
While the principles outlined above provide a general framework, some exceptions and nuances exist. Factors like:
-
Branching: The presence of branches in the fatty acid chain can influence packing efficiency and thus melting point. Branched fatty acids generally have lower melting points than their straight-chain counterparts.
-
Crystalline Structure: The way fatty acid molecules arrange themselves in a crystalline structure also influences the melting point. Different polymorphs (different crystalline forms) of the same fatty acid can have slightly different melting points.
-
Intermolecular Interactions Beyond van der Waals: While van der Waals forces are dominant, other weaker intermolecular interactions can play a subtle role, especially in complex mixtures of fatty acids.
Practical Applications and Conclusion
Understanding the relationship between fatty acid structure and melting point has significant practical applications. In the food industry, this knowledge is crucial for controlling the texture and consistency of food products. The melting point of fats and oils dictates their properties at different temperatures – influencing whether they are solid or liquid at room temperature. In the cosmetic and pharmaceutical industries, the melting points of fatty acids are critical for formulating creams, lotions, and ointments with the desired consistency.
In conclusion, arranging fatty acids in order of increasing melting point involves a systematic consideration of chain length, degree of saturation, and cis/trans isomerism. While general trends are predictable, some exceptions might occur due to factors like branching and crystalline structure. Mastering these principles is fundamental for anyone working in fields where the physical properties of fatty acids play a significant role. A deeper understanding provides valuable insights into the diverse applications and behavior of these essential molecules.
Latest Posts
Latest Posts
-
Hydrogen Is A Metal Nonmetal Or Metalloid
Mar 29, 2025
-
Equation Of Tangent Line Implicit Differentiation
Mar 29, 2025
-
According To The Bronsted Lowry Definition
Mar 29, 2025
-
What Are The Vertical Columns Called On A Periodic Table
Mar 29, 2025
-
Where Does Fermentation Take Place In A Cell
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Arrange The Fatty Acids In Order Of Increasing Melting Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
