Arrow Pushing To Convert Sugar Into Furanose Form
Muz Play
Mar 16, 2025 · 6 min read
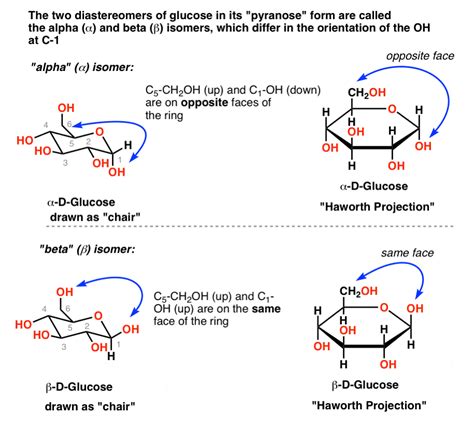
Table of Contents
Arrow Pushing to Convert Sugar into Furanose Form: A Deep Dive into Cyclization Mechanisms
Understanding carbohydrate chemistry is crucial in various fields, from biochemistry and medicine to food science and materials science. A key aspect of this understanding lies in the ability to predict and manipulate the different cyclic forms of sugars, particularly the conversion of open-chain forms into furanose structures. This article will delve into the intricacies of arrow pushing mechanisms involved in this conversion, providing a detailed, step-by-step explanation accessible to both beginners and experienced chemists. We will focus on the mechanism, exploring different reaction pathways and highlighting the importance of stereochemistry throughout the process.
Understanding the Players: Open-Chain Sugars and Furanose Rings
Before diving into the arrow-pushing mechanisms, let's establish a firm understanding of the molecules involved. Sugars, or monosaccharides, exist in both open-chain and cyclic forms. The open-chain form is an acyclic structure with multiple hydroxyl (-OH) groups and a carbonyl group (either an aldehyde or a ketone). This open-chain structure is in equilibrium with various cyclic forms.
The furanose form is a five-membered cyclic structure, named after the heterocyclic compound furan. The ring is formed by an intramolecular reaction between the carbonyl group and one of the hydroxyl groups, resulting in the formation of a hemiacetal (if the sugar started as an aldose) or hemiketal (if it started as a ketose) linkage. This ring closure significantly impacts the sugar's reactivity and properties.
Key Stereochemical Considerations
The formation of a furanose ring introduces new stereocenters. The carbonyl carbon in the open-chain form becomes chiral upon ring closure. This creates anomers, which are diastereomers differing only in the configuration at the anomeric carbon (the carbon that was part of the carbonyl group). These anomers are designated as α or β, depending on the orientation of the hydroxyl group at the anomeric carbon relative to the CH₂OH group on the furthest carbon from the anomeric carbon. α indicates that the hydroxyl group is on the opposite side (trans) to the CH₂OH group, while β indicates that it is on the same side (cis).
The Arrow-Pushing Mechanism: A Step-by-Step Guide
The conversion of an open-chain sugar into its furanose form involves a nucleophilic attack by a hydroxyl group on the carbonyl carbon. This reaction is an example of intramolecular hemiacetal or hemiketal formation. Let's visualize this mechanism using arrow pushing, focusing on a simple aldose, such as D-ribose, for clarity.
Step 1: Nucleophilic Attack
The oxygen atom of one of the hydroxyl groups acts as a nucleophile, attacking the electrophilic carbonyl carbon. This is shown by a curved arrow originating from the lone pair of electrons on the oxygen and pointing towards the carbonyl carbon.
O
||
HO-C-H
|
...
The double bond of the carbonyl group breaks, with one electron pair moving onto the oxygen atom, resulting in a negatively charged alkoxide ion.
Step 2: Proton Transfer
The negatively charged oxygen atom of the alkoxide ion then abstracts a proton from a nearby hydroxyl group or water molecule. This proton transfer is represented by another curved arrow moving from a hydroxyl group's hydrogen to the negatively charged oxygen.
OH
|
HO-C-H
|
...
This step neutralizes the charge, forming a hydroxyl group within the nascent ring structure.
Step 3: Ring Closure
With the formation of the new hydroxyl group, the ring is complete, forming the five-membered furanose ring. The carbons involved in the ring closure are now bonded in a cyclic structure, forming a hemiacetal.
O
/ \
/ \
/______\
The specific hydroxyl group that participates in the ring closure determines the configuration of the anomeric carbon, giving rise to α and β anomers.
Exploring Different Sugar Structures and their Furanose Forms
While we've used D-ribose as an example, the basic mechanism remains the same for other aldoses and ketoses. However, the specific stereochemistry and the resulting anomer configurations will vary based on the sugar's structure.
For example, D-fructose, a ketose, undergoes a similar ring-closure mechanism to form a furanose ring. The ketone group is attacked by a hydroxyl group, leading to the formation of a hemiketal linkage. The stereochemistry of the resulting furanose ring will be different compared to that formed from D-ribose. The complexity increases when dealing with larger monosaccharides with more chiral centers.
Factors Affecting Furanose Formation
Several factors influence the equilibrium between the open-chain form and the furanose (and pyranose) forms:
- Steric effects: The bulkiness of the substituents on the sugar molecule can influence the stability of the different cyclic forms. Less steric hindrance favours the formation of one anomer over another.
- Solvent effects: The solvent's polarity can also affect the equilibrium, favoring the more polar form in a polar solvent.
- Temperature: Temperature variations can influence the rate of ring closure and opening, affecting the proportions of the different forms.
Beyond the Basics: Applications and Further Considerations
The understanding of furanose formation is fundamental in many areas of chemistry and biology. The stability and reactivity of the furanose form significantly impact the properties and functions of many biologically important molecules:
- Nucleic acids: The ribose sugar in RNA exists primarily in the furanose form, and its specific configuration is critical for the structure and function of RNA.
- Enzyme activity: Many enzymes are highly specific to the furanose form of specific sugars, highlighting the importance of ring closure in biological processes.
- Drug design: Understanding sugar cyclization helps in designing drugs that target specific carbohydrate-binding proteins.
Challenges and Further Research
While the basic mechanisms are understood, several challenges remain in the field:
- Predicting anomer ratios: Accurately predicting the ratio of α and β anomers formed under specific conditions remains a challenging task.
- Understanding complex carbohydrate structures: The cyclization of complex carbohydrates and oligosaccharides introduces further complexities in understanding the mechanisms involved.
- Developing efficient methods for selective furanose formation: Developing new, efficient methods for selectively synthesizing specific furanose forms remains an active area of research.
Conclusion
The conversion of sugars into their furanose forms is a fundamental process in carbohydrate chemistry. The arrow-pushing mechanism provides a clear and concise way to understand the intramolecular nucleophilic attack and ring closure leading to hemiacetal/hemiketal formation. Understanding the intricacies of this mechanism, including stereochemical considerations and factors influencing the equilibrium between open-chain and cyclic forms, is crucial for advancing our knowledge in various scientific fields. Further research in this area will continue to unlock new possibilities in the design of new drugs, materials, and a deeper understanding of biological systems. The ability to manipulate and control sugar cyclization offers profound potential for future advancements in numerous scientific disciplines.
Latest Posts
Latest Posts
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Arrow Pushing To Convert Sugar Into Furanose Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
