Atomic Structure And The Periodic Table
Muz Play
Mar 28, 2025 · 7 min read
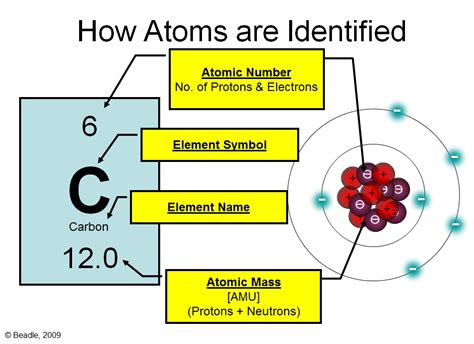
Table of Contents
Atomic Structure and the Periodic Table: A Deep Dive
The atom, the fundamental building block of matter, and the periodic table, its organized summary, are cornerstones of chemistry. Understanding their intricacies unlocks a deeper appreciation of the world around us, from the air we breathe to the technology we use. This comprehensive guide delves into the fascinating world of atomic structure and its representation in the periodic table, exploring their interconnectedness and significance.
Exploring the Atom: A Subatomic Journey
The atom, once considered indivisible (hence the name, from the Greek "atomos"), is now understood to be a complex system of subatomic particles. These particles, primarily protons, neutrons, and electrons, interact to define an atom's properties and behavior.
Protons: The Positive Charge Carriers
Protons reside within the atom's nucleus, a dense central region. Each proton carries a single positive electrical charge (+1). Crucially, the number of protons in an atom's nucleus, known as its atomic number, defines the element itself. Hydrogen, with one proton, is fundamentally different from helium, with two, and so on. This number is unique to each element and is unchangeable under normal chemical processes.
Neutrons: The Neutral Partners
Neutrons, also found in the nucleus, carry no electrical charge (hence "neutral"). Their presence contributes to an atom's mass but not its charge. Isotopes, atoms of the same element with varying numbers of neutrons, exist for many elements. While they have the same chemical properties due to the same number of protons, their physical properties can differ slightly due to the change in mass. For example, carbon-12 and carbon-14 are isotopes of carbon, with 6 and 8 neutrons respectively.
Electrons: The Orbiting Negatives
Electrons occupy the space surrounding the nucleus, existing in regions called electron shells or energy levels. These shells are not fixed orbits like planets around a sun but rather represent regions of probability where an electron is most likely to be found. Electrons carry a single negative electrical charge (-1), balancing the positive charge of the protons in a neutral atom. The arrangement of electrons in an atom's shells, specifically the number of electrons in the outermost shell (the valence electrons), dictates the atom's chemical reactivity.
Quantum Mechanics and Atomic Orbitals
The behavior of electrons within an atom isn't governed by classical physics but by the principles of quantum mechanics. Electrons don't simply orbit the nucleus in well-defined paths; instead, their positions are described by atomic orbitals, regions of space with a high probability of finding an electron. These orbitals have distinct shapes (s, p, d, f) and energy levels, influencing the atom's chemical properties and bonding capabilities. The filling of these orbitals follows specific rules, dictated by the Aufbau principle, the Pauli exclusion principle, and Hund's rule, which determine the electron configuration of an atom.
The Periodic Table: Organizing the Elements
The periodic table, a beautifully organized arrangement of elements, reflects the underlying patterns in atomic structure. Its structure is not arbitrary; it directly reflects the periodic trends in atomic properties, such as electronegativity, ionization energy, and atomic radius.
Organization by Atomic Number and Periodicity
Elements are arranged in increasing order of their atomic numbers. The table is structured into periods (horizontal rows) and groups (vertical columns). Elements within the same period have the same number of electron shells, while elements within the same group share similar valence electron configurations, leading to similar chemical properties. This recurring pattern of properties is known as periodicity.
Groups: Families of Elements
Groups, also called families, represent elements with similar chemical properties. For example, Group 1 (alkali metals) are highly reactive metals with one valence electron, while Group 18 (noble gases) are inert gases with full valence shells. The properties within a group are consistent because all elements share a similar electron configuration in their outermost shell.
Periods: Trends in Atomic Properties
Across a period, atomic properties exhibit trends. For example, atomic radius generally decreases as you move from left to right across a period, due to increasing nuclear charge pulling the electrons closer. Ionization energy, the energy required to remove an electron, generally increases across a period, as the increasing nuclear charge holds the electrons more tightly. Electronegativity, the tendency of an atom to attract electrons in a bond, also generally increases across a period.
Blocks: Subshells and Electron Configurations
The periodic table is also divided into blocks (s, p, d, f), representing the subshells being filled with electrons. The s-block elements are found in Groups 1 and 2, the p-block elements in Groups 13-18, the d-block elements (transition metals) in the middle of the table, and the f-block elements (lanthanides and actinides) at the bottom. These blocks reflect the filling of different atomic orbitals and contribute to the diverse properties observed across the table.
Special Cases and Anomalies
While the periodic table exhibits clear trends, some exceptions and anomalies exist. These deviations arise from the complex interplay of factors affecting electron configurations and atomic interactions, including electron shielding, electron-electron repulsion, and relativistic effects. Understanding these anomalies requires a deeper dive into quantum mechanics and atomic structure.
Atomic Structure and Chemical Bonding
The arrangement of electrons in an atom's outermost shell – the valence electrons – is the primary determinant of its chemical behavior and how it forms bonds with other atoms.
Ionic Bonding: Transfer of Electrons
Ionic bonding occurs when one atom transfers one or more electrons to another atom. This transfer creates ions: positively charged cations (formed by electron loss) and negatively charged anions (formed by electron gain). The electrostatic attraction between these oppositely charged ions forms the ionic bond. For example, sodium (Na) loses one electron to become Na+, and chlorine (Cl) gains one electron to become Cl-, forming the ionic compound sodium chloride (NaCl), or common table salt.
Covalent Bonding: Sharing Electrons
Covalent bonding involves the sharing of electrons between atoms. This sharing allows atoms to achieve a stable electron configuration, typically a full outer shell. Covalent bonds are crucial in the formation of molecules, and their strength influences the properties of the resulting compound. For example, the two hydrogen atoms in a hydrogen molecule (H2) share their electrons to form a stable covalent bond.
Metallic Bonding: A Sea of Electrons
Metallic bonding occurs in metals and involves a "sea" of delocalized electrons shared among many metal atoms. This creates a strong bond that accounts for metals' characteristic properties, such as high electrical conductivity and malleability.
Applications and Significance
Understanding atomic structure and the periodic table is fundamental to various scientific disciplines and technological advancements.
Chemistry and Material Science: Designing New Materials
The principles of atomic structure and bonding are crucial in designing new materials with specific properties. By carefully controlling the composition and arrangement of atoms, scientists can create materials with tailored characteristics for applications ranging from strong construction materials to highly efficient semiconductors.
Medicine and Biology: Understanding Biological Systems
Atomic structure is critical in understanding biological systems. The interactions of atoms in molecules such as proteins, DNA, and RNA determine their functions and play a vital role in biological processes. This knowledge is essential for drug discovery, diagnostics, and disease treatment.
Nuclear Science and Energy: Harnessing Atomic Power
Nuclear science relies heavily on understanding the structure of the atom's nucleus and how it can be manipulated. Nuclear fission and fusion, processes that involve changes in an atom's nucleus, are utilized in nuclear power generation and other applications.
Conclusion: A Continuous Journey of Discovery
The exploration of atomic structure and the periodic table is a continuously evolving field. As scientific tools and techniques improve, our understanding of these fundamental concepts becomes ever more refined. This journey of discovery not only deepens our understanding of the physical world but also drives technological innovations, impacting various aspects of our lives. From the development of new materials and medicines to advancements in energy production and environmental remediation, the principles of atomic structure and the periodic table remain central to scientific progress and technological advancements. The periodic table, far from being a static chart, serves as a dynamic roadmap guiding our exploration of the fundamental building blocks of matter and the universe itself.
Latest Posts
Latest Posts
-
What Are The Three General Characteristics Of Connective Tissue
Mar 31, 2025
-
Blocks Myosin Binding Sites On Actin
Mar 31, 2025
-
Label The Microscopic Structure Of A Skeletal Muscle
Mar 31, 2025
-
What Is The Difference Between Intermolecular Forces And Intramolecular Forces
Mar 31, 2025
-
Sampling With Replacement And Sampling Without Replacement
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Atomic Structure And The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
