Atomic Structure Of The Periodic Table
Muz Play
Mar 25, 2025 · 7 min read
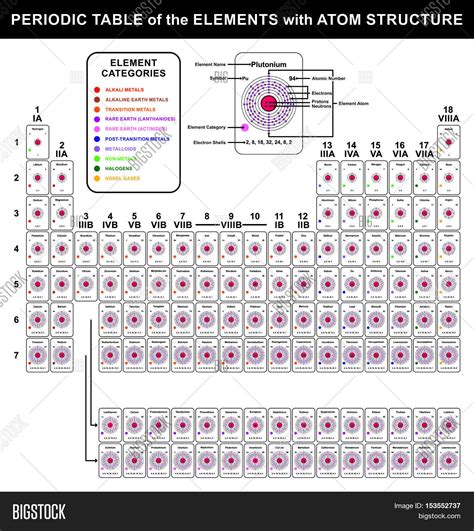
Table of Contents
Delving into the Atomic Structure Underlying the Periodic Table
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. Understanding the intricate details of atomic structure is crucial to comprehending the table's organization and the behavior of elements. This article delves deep into the atomic structure, exploring its fundamental components and how these components dictate an element's place and properties within the periodic table.
The Building Blocks: Subatomic Particles
Atoms, the fundamental units of matter, are composed of three primary subatomic particles: protons, neutrons, and electrons. These particles, though tiny, possess distinct properties that define an atom's characteristics.
1. Protons: The Positive Charge Carriers
Protons reside within the atom's nucleus, a dense central region. Each proton carries a single positive electrical charge (+1). The number of protons in an atom's nucleus, known as the atomic number, uniquely identifies the element. For instance, all hydrogen atoms have one proton (atomic number 1), all helium atoms have two (atomic number 2), and so on. The atomic number dictates an element's position on the periodic table, arranged in ascending order.
2. Neutrons: The Neutral Partners
Neutrons, also located in the nucleus, are electrically neutral, carrying no charge. Their mass is approximately equal to that of a proton. The number of neutrons in an atom's nucleus can vary, even within the same element. These variations are called isotopes. Isotopes of an element have the same number of protons but different numbers of neutrons, leading to variations in atomic mass. For example, carbon-12 and carbon-14 are isotopes of carbon; both have 6 protons, but carbon-12 has 6 neutrons, while carbon-14 has 8.
3. Electrons: The Orbiting Negatives
Electrons are negatively charged particles (-1) with a significantly smaller mass than protons or neutrons. They orbit the nucleus in specific energy levels, or shells, which are regions of space where there's a high probability of finding an electron. These shells are arranged in increasing distance from the nucleus. The outermost shell, known as the valence shell, plays a crucial role in determining an element's chemical reactivity. Electrons in the valence shell are called valence electrons. The number of valence electrons largely determines how an element will interact with other elements to form chemical bonds.
Electron Shells and Subshells: A Deeper Dive
The arrangement of electrons within an atom's shells and subshells is governed by quantum mechanics. Each shell can hold a maximum number of electrons, determined by the formula 2n², where 'n' is the principal quantum number representing the shell's energy level (n=1 for the first shell, n=2 for the second, and so on).
Shell Structure
- Shell 1 (n=1): Can hold a maximum of 2 electrons.
- Shell 2 (n=2): Can hold a maximum of 8 electrons.
- Shell 3 (n=3): Can hold a maximum of 18 electrons.
- Shell 4 (n=4): Can hold a maximum of 32 electrons.
And so on. The filling of these shells follows a specific pattern, influencing an element's chemical properties.
Subshell Structure
Within each shell, electrons occupy subshells designated by the letters s, p, d, and f. Each subshell has a specific shape and can hold a limited number of electrons:
- s subshell: Spherical shape, holds a maximum of 2 electrons.
- p subshell: Dumbbell shape, holds a maximum of 6 electrons.
- d subshell: More complex shape, holds a maximum of 10 electrons.
- f subshell: Even more complex shape, holds a maximum of 14 electrons.
The order of filling these subshells follows the Aufbau principle, which states that electrons fill the lowest energy levels first. This order is often represented by the diagonal rule or Aufbau diagram.
Atomic Structure and the Periodic Table: The Connection
The arrangement of electrons in an atom's outermost shell (valence shell) directly relates to its position and chemical behavior within the periodic table. Elements in the same group (vertical column) have the same number of valence electrons, leading to similar chemical properties.
Groups and Periods
- Groups (Columns): Elements within the same group share similar chemical properties because they have the same number of valence electrons. For example, Group 1 elements (alkali metals) all have one valence electron, making them highly reactive.
- Periods (Rows): Elements within the same period have the same number of electron shells. As you move across a period, the number of protons and electrons increases, leading to variations in atomic size and chemical properties.
Trends in the Periodic Table
The periodic table showcases various trends in atomic properties, directly linked to atomic structure:
- Atomic Radius: Generally increases down a group (due to added electron shells) and decreases across a period (due to increased nuclear charge).
- Ionization Energy: The energy required to remove an electron from an atom. Generally decreases down a group (due to increased atomic size) and increases across a period (due to increased nuclear charge).
- Electronegativity: An atom's ability to attract electrons in a chemical bond. Generally decreases down a group and increases across a period.
- Electron Affinity: The energy change when an electron is added to a neutral atom. Generally increases across a period and decreases down a group, with some exceptions.
These trends are fundamental to understanding chemical bonding and reactivity.
Beyond the Basics: Isotopes and Ions
Our understanding of atomic structure extends beyond the simple proton-neutron-electron model.
Isotopes: Variations on a Theme
As previously mentioned, isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. This leads to variations in atomic mass. Some isotopes are stable, while others are radioactive, undergoing nuclear decay. Radioactive isotopes have applications in various fields, including medicine and archaeology.
Ions: Charged Particles
Ions are atoms that have gained or lost electrons, resulting in a net electrical charge. Atoms that lose electrons become positively charged cations, while atoms that gain electrons become negatively charged anions. The formation of ions is crucial in many chemical reactions and plays a significant role in ionic bonding.
Advanced Concepts: Quantum Numbers and Electron Configurations
A complete description of atomic structure necessitates delving into quantum numbers and electron configurations.
Quantum Numbers
Quantum numbers provide a more precise description of an electron's state within an atom. Four quantum numbers are used:
- Principal Quantum Number (n): Describes the electron's energy level (shell).
- Azimuthal Quantum Number (l): Describes the electron's subshell (s, p, d, f).
- Magnetic Quantum Number (ml): Describes the electron's orbital within a subshell.
- Spin Quantum Number (ms): Describes the electron's intrinsic angular momentum (spin up or spin down).
These quantum numbers define the unique quantum state of each electron in an atom.
Electron Configurations
Electron configuration represents the arrangement of electrons in an atom's various shells and subshells. It follows the Aufbau principle and Hund's rule, which states that electrons will singly occupy orbitals within a subshell before pairing up. Electron configuration is essential for predicting an element's chemical properties and behavior. For instance, the electron configuration of an element can be used to determine its valence electrons and hence its group in the periodic table.
Conclusion: A Unified Framework
The atomic structure provides the fundamental framework for understanding the periodic table and the behavior of elements. From the simple model of protons, neutrons, and electrons to the intricate details of quantum numbers and electron configurations, a comprehensive understanding of atomic structure is crucial for advancements in chemistry, physics, and materials science. The periodic table, with its seemingly simple organization, reveals a profound depth of atomic complexity, showcasing the elegance and power of scientific principles. Further exploration into these concepts reveals a deeper appreciation for the interconnectedness of matter and the fundamental laws that govern its behavior. This continuous pursuit of knowledge paves the way for future discoveries and advancements in our understanding of the universe.
Latest Posts
Latest Posts
-
On The Weak Acid Strong Base Titration Curve
Mar 28, 2025
-
Label The Cranial Dura Septa In The Figure
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Atomic Structure Of The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
