Atoms Share Electrons Unequally S An Blank Bond
Muz Play
Mar 16, 2025 · 6 min read
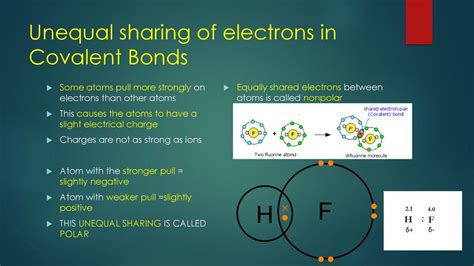
Table of Contents
Atoms Share Electrons Unequally: An Introduction to Polar Bonds
Atoms are the fundamental building blocks of matter, and their interactions determine the properties of molecules and materials. One crucial type of interaction involves the sharing of electrons between atoms to form chemical bonds. While some bonds involve equal sharing, many others exhibit unequal electron sharing, resulting in what we call polar bonds. Understanding polar bonds is fundamental to comprehending the behavior of molecules and their interactions. This in-depth article will explore the nature of polar bonds, their consequences, and their role in various chemical phenomena.
What is a Polar Bond?
A polar bond, also known as a polar covalent bond, is a covalent bond between two atoms where the electrons forming the bond are shared unequally. This unequal sharing arises due to differences in the electronegativity of the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Atoms with higher electronegativity exert a stronger pull on the shared electrons, drawing them closer to their nucleus.
The Role of Electronegativity
The difference in electronegativity between two atoms is the key factor determining the polarity of a bond. When two atoms with significantly different electronegativities form a bond, the more electronegative atom will acquire a partial negative charge (δ-), while the less electronegative atom will acquire a partial positive charge (δ+). This separation of charges creates a dipole moment, a vector quantity representing the magnitude and direction of the charge separation. The greater the difference in electronegativity, the larger the dipole moment and the more polar the bond.
Contrast with Nonpolar Covalent Bonds
It's important to contrast polar bonds with nonpolar covalent bonds. In nonpolar covalent bonds, the electrons are shared relatively equally between the two atoms. This typically occurs when the atoms involved have similar electronegativities, such as in a bond between two identical atoms (e.g., H₂ or O₂). In these cases, the dipole moment is essentially zero.
Factors Influencing Polar Bond Formation
Several factors contribute to the formation of polar bonds:
1. Electronegativity Differences: The Primary Factor
The most crucial factor influencing polar bond formation is the difference in electronegativity between the atoms involved. A large electronegativity difference leads to a significant charge separation and a strongly polar bond. The Pauling electronegativity scale provides a quantitative measure of electronegativity, allowing for the prediction of bond polarity. For instance, a large electronegativity difference between oxygen (high electronegativity) and hydrogen (low electronegativity) results in the highly polar O-H bond found in water (H₂O).
2. Atomic Size: A Secondary Influence
While electronegativity is the primary determinant, atomic size also plays a secondary role. Larger atoms tend to have lower electronegativity because their valence electrons are further from the nucleus and less tightly held. Consequently, larger atoms are less effective at attracting shared electrons. This effect is often less pronounced than the electronegativity difference but can contribute to the overall bond polarity.
3. Bond Length: Impact on Dipole Moment
The length of the bond also influences the dipole moment. A shorter bond generally leads to a stronger dipole moment because the charges are closer together. Conversely, longer bonds can result in weaker dipole moments due to increased distance between the partial charges.
Consequences of Polar Bonds
The unequal sharing of electrons in polar bonds has several important consequences:
1. Molecular Dipole Moment: Creating Polar Molecules
The presence of polar bonds often leads to a molecular dipole moment. This is the overall dipole moment of the molecule, considering the individual bond dipole moments and their spatial arrangement. If the individual bond dipoles cancel each other out due to symmetry (e.g., in CO₂), the molecule will be nonpolar despite having polar bonds. However, if the individual bond dipoles do not cancel out (e.g., in H₂O), the molecule will possess a net dipole moment and be considered a polar molecule.
2. Solubility and Intermolecular Forces: Dictating Physical Properties
Polar molecules exhibit different physical properties than nonpolar molecules due to the presence of a dipole moment. Polar molecules are generally more soluble in polar solvents (like water) due to the strong electrostatic interactions between the dipoles. They also exhibit stronger intermolecular forces, such as dipole-dipole interactions and hydrogen bonding, leading to higher boiling points and melting points compared to nonpolar molecules with similar molecular weights.
3. Reactivity: Influencing Chemical Behavior
The partial charges in polar bonds make molecules more reactive. The partially positive atom is more susceptible to nucleophilic attack (attack by electron-rich species), while the partially negative atom is more susceptible to electrophilic attack (attack by electron-deficient species). This difference in reactivity is crucial in many chemical reactions, including those in organic chemistry and biochemistry.
4. Spectroscopic Properties: Observable Consequences
Polar bonds have characteristic spectroscopic properties. Techniques like infrared (IR) spectroscopy can detect the presence of polar bonds by observing the absorption of IR radiation due to vibrational modes of the bond. The frequency and intensity of absorption are related to the bond strength and polarity.
Examples of Polar Bonds and Molecules
Many common molecules contain polar bonds:
-
Water (H₂O): The O-H bonds are highly polar due to the large electronegativity difference between oxygen and hydrogen. The bent molecular geometry prevents the bond dipoles from canceling out, resulting in a polar molecule.
-
Ammonia (NH₃): The N-H bonds are polar, and the pyramidal geometry of the molecule contributes to its overall polarity.
-
Hydrogen Fluoride (HF): The H-F bond is extremely polar due to the high electronegativity of fluorine.
-
Carbonyl Groups (C=O): The C=O bond is polar due to the greater electronegativity of oxygen. This polarity plays a crucial role in the reactivity of carbonyl compounds.
-
Hydroxyl Groups (-OH): The O-H bond is polar and contributes to the properties of alcohols and other organic molecules containing hydroxyl groups.
Applications and Significance
The understanding of polar bonds has profound implications across numerous scientific disciplines:
1. Chemistry: Understanding Reactivity and Reactions
Polarity is a fundamental concept in understanding chemical reactivity. The partial charges in polar bonds dictate how molecules interact and react with each other. This knowledge is essential in designing and predicting the outcome of chemical reactions, synthesis of new molecules, and development of new materials.
2. Biochemistry: Essential for Biological Processes
Polar bonds are crucial for the structure and function of biological molecules. Proteins, DNA, and carbohydrates all contain numerous polar bonds, which influence their folding, interactions, and biological activity. For example, hydrogen bonding between polar groups is essential for the stability of the DNA double helix.
3. Materials Science: Developing New Materials
The understanding of polar bonds allows materials scientists to design and synthesize materials with specific properties. By manipulating the polarity of molecules and their interactions, they can create materials with tailored electrical, optical, and mechanical characteristics. Polar materials find applications in electronics, optics, and energy storage.
4. Environmental Science: Understanding Environmental Interactions
Polarity plays a critical role in the behavior of pollutants in the environment. The solubility and reactivity of pollutants are often determined by their polarity. Understanding this relationship is essential for assessing environmental risks and developing effective remediation strategies.
Conclusion: The Importance of Polar Bonds
Polar bonds are a fundamental aspect of chemical bonding with far-reaching consequences. The unequal sharing of electrons, driven by electronegativity differences, leads to a wide range of chemical and physical properties. Understanding polar bonds is crucial in many scientific fields, including chemistry, biochemistry, materials science, and environmental science. From predicting chemical reactivity to designing new materials, the concept of polar bonds remains a cornerstone of our understanding of the molecular world. Continued research and exploration of polar bonds will undoubtedly lead to further advances in these fields and beyond.
Latest Posts
Latest Posts
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
-
Electric Potential From A Point Charge
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Atoms Share Electrons Unequally S An Blank Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
