Axial And Equatorial Positions In Cyclohexane
Muz Play
Mar 17, 2025 · 5 min read
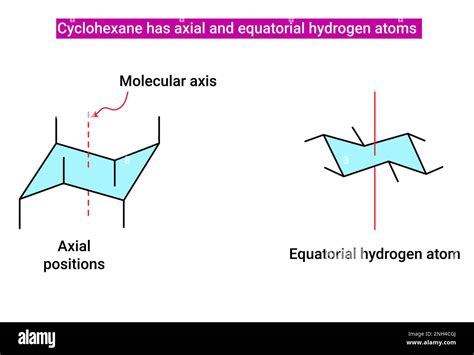
Table of Contents
Axial and Equatorial Positions in Cyclohexane: A Comprehensive Guide
Cyclohexane, a seemingly simple molecule with the formula C₆H₁₂, holds a fascinating complexity in its conformational analysis. Understanding its different conformations, specifically the axial and equatorial positions of its substituents, is crucial for predicting reactivity, stability, and physical properties of substituted cyclohexanes. This detailed guide will delve into the intricacies of axial and equatorial positions, exploring their implications in organic chemistry.
Understanding Cyclohexane Conformations
Before diving into axial and equatorial positions, let's establish a foundational understanding of cyclohexane's conformations. Cyclohexane doesn't exist as a flat, planar hexagon. The ideal bond angle for carbon is 109.5°, and a planar hexagon would force angles of 120°, creating significant strain. To alleviate this angle strain, cyclohexane adopts various conformations, constantly flexing and changing between them.
Two key conformations are prominent:
1. Chair Conformation: The Most Stable
The chair conformation is the most stable conformation of cyclohexane. In this arrangement, the molecule resembles a chair, with six carbon atoms forming a puckered structure. This conformation minimizes both angle strain and torsional strain (strain arising from eclipsing interactions between hydrogen atoms).
2. Boat Conformation: Less Stable
The boat conformation is considerably less stable than the chair conformation. In this conformation, the molecule resembles a boat. This arrangement introduces significant steric interactions between the flagpole hydrogens (those pointing upwards), increasing the energy of this conformation. A slightly more stable variation is the twist-boat conformation, where some of the steric interactions are relieved by a slight twist in the structure. However, the chair conformation remains significantly more stable.
Axial and Equatorial Positions: Defining the Difference
The chair conformation introduces two distinct types of positions for substituents: axial and equatorial. Understanding these positions is crucial for predicting the properties of substituted cyclohexanes.
Axial Positions: Vertical Alignment
Axial positions are those where the substituents are aligned vertically, parallel to the axis of the ring. There are six axial positions in a cyclohexane chair conformation – three pointing up and three pointing down. These positions experience significant 1,3-diaxial interactions, a type of steric hindrance.
Equatorial Positions: Around the Equator
Equatorial positions are those where the substituents are aligned approximately horizontally, around the "equator" of the ring. There are also six equatorial positions, alternating with the axial positions. Substituents in equatorial positions generally experience less steric hindrance than those in axial positions.
Steric Hindrance and 1,3-Diaxial Interactions
The major factor influencing the stability of substituted cyclohexanes is steric hindrance. This refers to the repulsive interactions between atoms or groups that are close together in space. In the case of cyclohexane, the most significant steric hindrance arises from 1,3-diaxial interactions.
These interactions occur between an axial substituent and the two axial hydrogens (or other substituents) on the carbons three atoms away. These interactions increase the energy of the molecule, making conformations with bulky substituents in axial positions less stable.
Conformational Equilibrium and Anomer Effects
Substituted cyclohexanes exist in an equilibrium between two chair conformations. Interconversion between these conformations occurs by ring flipping, where one chair conformation inverts to the other. However, the equilibrium is not always 50/50. The relative stability of the two conformations depends on the size and nature of the substituent.
Bulky substituents strongly prefer to occupy the equatorial positions. This preference minimizes 1,3-diaxial interactions and leads to a greater population of the conformation with the substituent in the equatorial position. This is often expressed as the equilibrium constant for the two chair forms.
Anomeric Effect: A Special Case
A notable exception to the simple steric hindrance rule is the anomeric effect. This phenomenon is observed in cyclic systems containing an electronegative atom (like oxygen in a pyranose ring) adjacent to a carbon with a substituent. In this situation, an axial substituent might be favored due to orbital interactions, overriding the expected steric preference for an equatorial position. This effect is particularly important in carbohydrate chemistry.
Predicting Stability and Reactivity
The principles of axial and equatorial positions are fundamental to predicting the stability and reactivity of substituted cyclohexanes. Consider the following:
-
Stability: Conformations with bulky substituents in equatorial positions are significantly more stable than those with the same substituents in axial positions. This directly relates to the minimization of steric hindrance.
-
Reactivity: The orientation of a substituent (axial or equatorial) can dramatically influence its reactivity. For instance, in reactions involving backside attack, axial substituents are more accessible to nucleophiles or electrophiles. Equatorial substituents are sterically hindered, making them less reactive in such reactions.
Applications and Examples
The concepts of axial and equatorial positions have wide-ranging applications in organic chemistry:
-
Conformational analysis: Predicting the most stable conformation of molecules is crucial in understanding their properties and reactivity.
-
Stereochemistry: Axial and equatorial positions are critical in determining the stereochemistry of reactions involving cyclohexane derivatives.
-
Drug design: The spatial arrangement of substituents in drug molecules influences their binding affinity to target receptors. Understanding axial and equatorial positions is vital in designing drugs with optimal binding.
-
Polymer chemistry: The conformation of cyclohexane rings in polymers impacts their physical properties, such as flexibility and strength.
-
Carbohydrate chemistry: The anomeric effect plays a significant role in the conformations and properties of carbohydrates.
Advanced Concepts and Further Exploration
While this article covers the fundamentals of axial and equatorial positions in cyclohexane, several advanced concepts deserve further exploration:
-
Complex substituents: The impact of multiple substituents and their interactions on conformational equilibrium becomes increasingly complex with more substituents. Methods like the A-value concept are used to predict the conformational preferences.
-
Ring-flipping kinetics: The rate of interconversion between chair conformations can be influenced by several factors.
-
Computational chemistry: Advanced computational methods provide a powerful tool for investigating cyclohexane conformations and their energies, allowing for more precise predictions.
Conclusion
Axial and equatorial positions in cyclohexane are fundamental concepts in organic chemistry with profound implications for the stability, reactivity, and physical properties of a wide range of molecules. Understanding the concepts of steric hindrance, 1,3-diaxial interactions, and the anomeric effect is crucial for predicting the behavior of substituted cyclohexanes and interpreting experimental data. This detailed exploration has provided a comprehensive overview, equipping readers with a strong foundation in this critical area of organic chemistry. The principles discussed here are fundamental to numerous fields, highlighting the importance of grasping this seemingly simple yet profoundly impactful concept. Further exploration into more advanced topics within this field will undoubtedly reveal even greater depth and complexity.
Latest Posts
Latest Posts
-
Limiting Reactant And Percent Yield Lab
Mar 17, 2025
-
What Are The Four Agents Of Socialization
Mar 17, 2025
-
What Do Humans Need To Survive
Mar 17, 2025
-
How Many Covalent Bonds Does Oxygen Have
Mar 17, 2025
-
Identify The Equation For The Graph
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Axial And Equatorial Positions In Cyclohexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
