Ba Oh 2 Strong Or Weak
Muz Play
Mar 22, 2025 · 5 min read
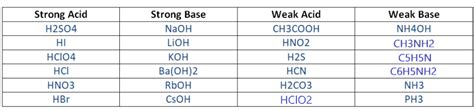
Table of Contents
- Ba Oh 2 Strong Or Weak
- Table of Contents
- Ba(OH)₂: Strong or Weak Base? Understanding its Properties and Applications
- Understanding the Concept of Strong and Weak Bases
- Ba(OH)₂: A Strong Base
- Factors Affecting Ba(OH)₂'s Strength
- Solubility of Barium Hydroxide: A Complication
- Applications of Barium Hydroxide
- 1. Industrial Applications:
- 2. Laboratory Applications:
- 3. Other Uses:
- Safety Precautions when Handling Ba(OH)₂
- Conclusion: Ba(OH)₂ - A Strong Base with Limited Solubility
- Latest Posts
- Latest Posts
- Related Post
Ba(OH)₂: Strong or Weak Base? Understanding its Properties and Applications
Barium hydroxide, Ba(OH)₂, is a common inorganic compound frequently encountered in chemistry. A crucial question often arises: is Ba(OH)₂ a strong or weak base? The answer is crucial for understanding its behavior in chemical reactions and its various applications. This comprehensive article will delve into the properties of Ba(OH)₂, explore its classification as a strong base, examine its solubility, and discuss its numerous applications in different fields.
Understanding the Concept of Strong and Weak Bases
Before classifying Ba(OH)₂, let's revisit the fundamental difference between strong and weak bases. A strong base is a base that completely dissociates into its ions (cations and anions) in an aqueous solution. This means that every molecule of the strong base breaks apart into its constituent ions. Conversely, a weak base only partially dissociates, meaning that only a small fraction of the molecules break into ions, while the majority remains undissociated. The degree of dissociation determines the strength of the base.
This difference is reflected in the equilibrium constant for the base dissociation reaction. Strong bases have a very large equilibrium constant (Kb), indicating a high degree of dissociation. Weak bases have a smaller Kb value, reflecting a lower degree of dissociation.
Ba(OH)₂: A Strong Base
Barium hydroxide, Ba(OH)₂, is unequivocally categorized as a strong base. When dissolved in water, it completely dissociates into barium cations (Ba²⁺) and hydroxide anions (OH⁻):
Ba(OH)₂(s) → Ba²⁺(aq) + 2OH⁻(aq)
This complete dissociation is the hallmark of a strong base. The presence of a high concentration of hydroxide ions (OH⁻) in the solution is what makes it strongly alkaline, resulting in a high pH value.
Factors Affecting Ba(OH)₂'s Strength
Several factors contribute to Ba(OH)₂'s classification as a strong base:
-
Ionic Character: The strong ionic character of the Ba-OH bond facilitates complete dissociation in water. The electrostatic attraction between the highly charged Ba²⁺ cation and the OH⁻ anion is relatively weak, allowing for easy separation in a polar solvent like water.
-
High Solubility (to a degree): While barium hydroxide's solubility is not unlimited, its relatively high solubility in water ensures that a significant amount of the compound dissociates, generating a high concentration of OH⁻ ions. This high concentration of hydroxide ions directly contributes to its strong basicity. (We will discuss solubility in more detail later).
-
Lack of Back-bonding: Unlike some weak bases which exhibit back-bonding (where electrons are donated back to the central atom), Ba(OH)₂ lacks such interactions. This absence of back-bonding further facilitates complete dissociation.
Solubility of Barium Hydroxide: A Complication
While Ba(OH)₂ is a strong base in terms of its complete dissociation, its solubility in water is a crucial consideration. Unlike other strong bases like NaOH or KOH, which are highly soluble, Ba(OH)₂ has limited solubility. This means that even though the dissolved portion completely dissociates, the overall amount of hydroxide ions produced might be less compared to highly soluble strong bases.
However, it's crucial to remember that solubility and strength are distinct properties. The amount of Ba(OH)₂ that does dissolve completely dissociates; it's not partially dissociated like a weak base. Therefore, the dissolved portion behaves as a strong base.
The limited solubility of Ba(OH)₂ is due to the relatively strong lattice energy of its crystalline structure. Breaking apart the Ba(OH)₂ crystal lattice requires a significant amount of energy.
Applications of Barium Hydroxide
Despite its limited solubility, barium hydroxide finds application in various fields:
1. Industrial Applications:
-
Sugar Refining: Ba(OH)₂ is used in the refining of sugar beet to remove impurities. Its strong basicity helps to precipitate undesirable substances, leading to a purer sugar product.
-
Chemical Synthesis: Ba(OH)₂ serves as a starting material or reagent in various chemical syntheses. Its strong base properties are utilized in reactions requiring deprotonation or neutralization.
-
Wastewater Treatment: Ba(OH)₂ can be used in wastewater treatment to neutralize acidic solutions or precipitate certain contaminants. Its ability to remove heavy metals is a key benefit.
2. Laboratory Applications:
-
Titrations: Ba(OH)₂ solutions, while less commonly used than NaOH or KOH due to solubility issues, can be employed as a titrant in acid-base titrations. Careful preparation and standardization are essential to ensure accurate results.
-
Preparation of Other Barium Compounds: Ba(OH)₂ serves as a precursor for the preparation of other barium compounds. This is due to the ease with which the barium ion can be exchanged in reactions.
3. Other Uses:
-
Grease Removal: Due to its strong alkaline nature, Ba(OH)₂ is used in certain industrial grease removal processes.
-
Lubricant Additives: Barium hydroxide is sometimes used as an additive in lubricating greases, acting as a stabilizer.
Safety Precautions when Handling Ba(OH)₂
Barium hydroxide, like many strong bases, is a corrosive substance. Appropriate safety precautions must always be taken when handling it:
-
Eye Protection: Always wear safety glasses or goggles when handling Ba(OH)₂ to prevent accidental eye contact. If contact occurs, immediately flush eyes with copious amounts of water and seek medical attention.
-
Skin Protection: Wear gloves and protective clothing to prevent skin contact. If skin contact occurs, wash the affected area thoroughly with water and soap.
-
Respiratory Protection: Use appropriate respiratory protection in case of dust or aerosols to prevent inhalation.
-
Disposal: Dispose of barium hydroxide waste according to local regulations and safety guidelines.
Conclusion: Ba(OH)₂ - A Strong Base with Limited Solubility
Barium hydroxide, Ba(OH)₂, is undeniably a strong base due to its complete dissociation in water. However, its limited solubility distinguishes it from other highly soluble strong bases such as sodium hydroxide (NaOH) and potassium hydroxide (KOH). Understanding this crucial distinction between strength and solubility is essential for its safe and effective use in various industrial and laboratory applications. While the dissolved portion acts as a potent strong base, the overall concentration of hydroxide ions needs to be accounted for due to the solubility limitations. Always prioritize safety when handling Ba(OH)₂ because of its corrosive nature. This detailed understanding of barium hydroxide's properties and applications allows for its responsible and effective utilization across multiple sectors. Remember that safety precautions are paramount when dealing with this strong base.
Latest Posts
Latest Posts
-
Are Metals On The Right Side O The Periodic Table
Mar 24, 2025
-
The Chromosome Theory Of Inheritance States That
Mar 24, 2025
-
Symmetric With Respect To The Y Axis
Mar 24, 2025
-
Classifying Matter Using Particle Models 2
Mar 24, 2025
-
What Is The Horizontal Rows On The Periodic Table Called
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Ba Oh 2 Strong Or Weak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
