Boiling Water Physical Or Chemical Change
Muz Play
Mar 18, 2025 · 5 min read
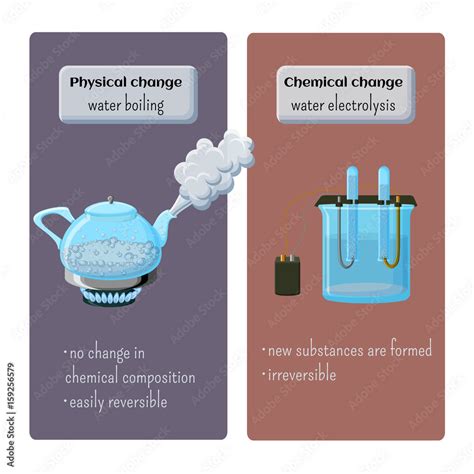
Table of Contents
Boiling Water: A Physical Change Explained
Is boiling water a physical or chemical change? This seemingly simple question delves into the fundamental concepts of matter and its transformations. Understanding the difference between physical and chemical changes is crucial in various scientific fields, from chemistry and physics to cooking and everyday observations. This article will delve deep into the process of boiling water, explaining why it's classified as a physical change, exploring the underlying scientific principles, and debunking common misconceptions.
Understanding Physical and Chemical Changes
Before we dissect the boiling of water, let's clarify the core distinctions between physical and chemical changes.
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. Think of it as rearranging the molecules without breaking or forming new bonds. Examples include:
- Changes in state: Melting ice, freezing water, boiling water, condensing steam – these all involve changes in the physical state (solid, liquid, gas) but the water molecules remain H₂O.
- Shape changes: Crushing a can, cutting paper, bending a wire – these alter the shape but not the material's chemical makeup.
- Dissolving: Salt dissolving in water is a physical change because the salt molecules are dispersed in the water, but the salt and water molecules themselves remain unchanged. They can be separated through evaporation.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the formation of new substances with different chemical properties. This happens through the breaking and forming of chemical bonds. Indicators of a chemical change include:
- Formation of a gas: Bubbles forming, a change in odor.
- Formation of a precipitate: The formation of a solid from a solution.
- Color change: A significant and unexpected color change often suggests a chemical reaction.
- Temperature change: A significant temperature change, either an increase (exothermic) or decrease (endothermic), may indicate a chemical reaction.
- Irreversible change: Many chemical changes are irreversible or require significant energy input to reverse.
Boiling Water: A Deep Dive
Now, let's focus on the process of boiling water. When water boils, it transitions from a liquid state to a gaseous state (steam). This is a phase transition, a type of physical change. The water molecules themselves remain H₂O; they haven't transformed into a different substance.
The Molecular Perspective
At the molecular level, boiling water involves an increase in the kinetic energy of water molecules. As heat is applied, the molecules move faster and faster. Eventually, they gain enough kinetic energy to overcome the intermolecular forces (hydrogen bonds) holding them together in the liquid state. This allows them to escape the liquid's surface and transition into the gaseous phase (steam).
Key Characteristics of Boiling Water as a Physical Change
- No new substance is formed: The chemical formula remains H₂O throughout the process. Steam is still water, just in a different state.
- Reversible process: By cooling the steam, you can condense it back into liquid water. This demonstrates the reversibility characteristic of a physical change.
- No significant energy changes (excluding latent heat): While energy is required to heat the water and cause the phase change, this energy input doesn't create new chemical bonds. The energy is used to overcome intermolecular forces. The latent heat of vaporization represents the energy needed to change state, not to change chemical composition.
- Separation is possible: Steam can be condensed back into liquid water. This proves that no chemical reaction has taken place.
Debunking Common Misconceptions
Several misconceptions surround boiling water and its classification. Let's address some common ones:
Misconception 1: Bubbles are a sign of a chemical change.
Reality: The bubbles observed during boiling are primarily steam (water vapor). They are formed because the water molecules have gained enough kinetic energy to escape the liquid phase and transition into gas. This is a physical change, not a chemical reaction. While some dissolved gases might be released, the dominant component of the bubbles is water vapor.
Misconception 2: A change in state always implies a chemical change.
Reality: Changes in state (solid, liquid, gas) are almost always physical changes. They involve the rearrangement of molecules due to changes in temperature or pressure, not the formation of new substances.
Misconception 3: Water changes its properties entirely when boiled.
Reality: While the properties change (density, viscosity), the fundamental chemical composition remains the same. Water remains H₂O; it's just in a different physical state.
Beyond Boiling: Other Physical Changes of Water
Water undergoes many physical changes besides boiling:
- Melting: Ice melting into water.
- Freezing: Water freezing into ice.
- Sublimation: Ice transforming directly into water vapor (without passing through the liquid phase). This occurs at temperatures below the freezing point but at low pressures.
- Condensation: Water vapor transforming into liquid water.
These are all examples of physical changes as the water molecules retain their chemical identity, H₂O.
Practical Applications and Conclusion
The understanding that boiling water is a physical change has broad practical applications:
- Cooking: Boiling water is crucial in cooking processes, from pasta to vegetables. Knowing that it's a physical change helps us control the temperature and time effectively.
- Distillation: Distillation uses the boiling and condensation of water to purify it, separating it from dissolved impurities.
- Steam generation: The generation of steam is a critical process in power plants, utilizing the boiling of water to create energy.
- Scientific experimentation: Understanding phase transitions is crucial in various scientific experiments and applications involving material science and thermodynamics.
In conclusion, boiling water is unequivocally a physical change. It involves a change in the physical state of water from liquid to gas, but the chemical composition of water (H₂O) remains unchanged throughout the process. Understanding this distinction is critical for comprehending fundamental scientific principles and applying them in various practical situations. The process is reversible, no new substances are created, and the chemical identity of water persists. This simple example highlights the importance of distinguishing between physical and chemical changes in our everyday lives and scientific endeavors. Remember that a change in state, like boiling, does not inherently imply a chemical transformation.
Latest Posts
Latest Posts
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
-
Difference Between A Strong And Weak Acid
Mar 18, 2025
-
What Are The Five Evaluation Criteria
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
