Buffer Solution Of Acetic Acid And Sodium Acetate
Muz Play
Mar 31, 2025 · 6 min read
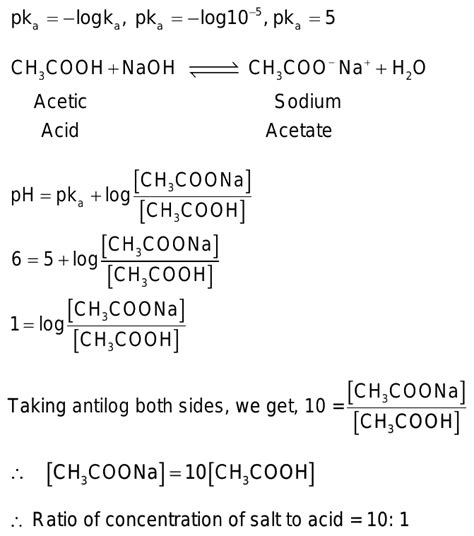
Table of Contents
Understanding Buffer Solutions: A Deep Dive into Acetic Acid and Sodium Acetate
Buffer solutions are crucial in various chemical and biological systems, maintaining a relatively stable pH even when small amounts of acid or base are added. This article will delve into the fascinating world of buffer solutions, focusing specifically on the acetic acid/sodium acetate buffer system. We'll explore its properties, preparation, applications, and the underlying chemistry that makes it so effective.
What is a Buffer Solution?
A buffer solution, also known as a pH buffer, is an aqueous solution that resists changes in pH upon the addition of small amounts of acid or base. This resistance to pH change is a key characteristic, making buffers indispensable in many applications. They achieve this stability through the presence of a weak acid and its conjugate base (or a weak base and its conjugate acid).
How Buffer Solutions Work: The Equilibrium Principle
The magic behind buffer solutions lies in the equilibrium established between the weak acid and its conjugate base. Consider the acetic acid/sodium acetate buffer:
- Acetic acid (CH₃COOH) is a weak acid, meaning it only partially dissociates in water.
- Sodium acetate (CH₃COONa) is the salt of acetic acid, providing the conjugate base, acetate ion (CH₃COO⁻).
The equilibrium reaction is:
CH₃COOH(aq) ⇌ CH₃COO⁻(aq) + H⁺(aq)
When a small amount of strong acid (like HCl) is added, the added H⁺ ions react with the acetate ions (CH₃COO⁻) to form more acetic acid (CH₃COOH), minimizing the change in pH. Conversely, when a small amount of strong base (like NaOH) is added, the hydroxide ions (OH⁻) react with the acetic acid (CH₃COOH) to form more acetate ions (CH₃COO⁻) and water, again mitigating the pH change.
The Acetic Acid/Sodium Acetate Buffer System: A Detailed Look
The acetic acid/sodium acetate buffer system is a classic example, widely used and easily understood. Its effectiveness stems from the readily available components and the relatively simple chemistry involved.
Strengths and Weaknesses of the System
Strengths:
- Readily Available: Both acetic acid and sodium acetate are inexpensive and readily available chemicals.
- Easy to Prepare: The buffer solution is straightforward to prepare, requiring simple mixing and measuring.
- Effective pH Range: This buffer system is most effective within a pH range of approximately 3.76 to 5.76, making it suitable for many applications requiring this pH range. The specific pH depends on the ratio of acetic acid to sodium acetate.
- Relatively Stable: The buffer solution maintains its pH stability over a reasonable period, especially if stored properly.
Weaknesses:
- Limited pH Range: The effective buffering capacity is limited to the pH range mentioned above. Outside this range, its effectiveness significantly decreases.
- Temperature Sensitivity: The pH of the buffer solution can be slightly affected by temperature changes.
- Susceptibility to Microbial Growth: Acetic acid solutions can be susceptible to microbial growth over time, especially if not stored properly.
Calculating the pH of an Acetic Acid/Sodium Acetate Buffer: The Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a crucial tool for calculating the pH of a buffer solution. For the acetic acid/sodium acetate buffer, it takes the form:
pH = pKa + log([CH₃COO⁻]/[CH₃COOH])
Where:
- pH: The pH of the buffer solution.
- pKa: The negative logarithm of the acid dissociation constant (Ka) of acetic acid (approximately 4.76 at 25°C).
- [CH₃COO⁻]: The concentration of the acetate ion (from sodium acetate).
- [CH₃COOH]: The concentration of acetic acid.
This equation highlights the importance of the ratio of conjugate base to weak acid in determining the buffer's pH.
Preparing an Acetic Acid/Sodium Acetate Buffer Solution
Preparing an acetic acid/sodium acetate buffer is a relatively simple process:
- Determine the Desired pH: Decide on the specific pH you need for your application within the effective range of the buffer.
- Calculate the Required Concentrations: Use the Henderson-Hasselbalch equation to calculate the required concentrations of acetic acid and sodium acetate to achieve the desired pH.
- Weigh and Measure: Accurately weigh out the calculated masses of acetic acid and sodium acetate.
- Dissolve in Water: Dissolve the weighed chemicals in a suitable volume of distilled water.
- Adjust the pH (if necessary): Use a pH meter to check the pH of the solution and make minor adjustments with small additions of acid or base if needed.
- Adjust the Final Volume: Add more distilled water to bring the solution to the desired final volume.
Applications of the Acetic Acid/Sodium Acetate Buffer
The acetic acid/sodium acetate buffer system finds applications in a variety of fields:
1. Biochemistry and Biology
- Enzyme Assays: Maintaining a stable pH is crucial for enzyme activity. This buffer helps ensure consistent enzyme activity during experiments.
- Cell Culture: Many cell cultures require a specific pH range for optimal growth, and this buffer helps maintain that environment.
- Protein Purification: The buffer is used in various protein purification techniques to maintain the protein's stability and functionality.
2. Analytical Chemistry
- Titrations: Buffer solutions are often used in titrations to maintain a stable pH during the addition of titrant.
- Electrochemistry: Buffers are essential in many electrochemical experiments where pH stability is crucial for accurate measurements.
3. Industrial Applications
- Textile Industry: pH control is vital in textile dyeing and finishing processes.
- Food Industry: This buffer helps maintain the desired pH in certain food products.
- Pharmaceutical Industry: Maintaining a stable pH is important for drug formulation and stability.
Factors Affecting Buffer Capacity
Buffer capacity refers to the amount of acid or base a buffer can neutralize before a significant change in pH occurs. Several factors influence buffer capacity:
- Concentration of the Buffer Components: Higher concentrations of both the weak acid and its conjugate base lead to a greater buffer capacity.
- Ratio of Acid to Conjugate Base: The buffer capacity is highest when the concentrations of the weak acid and its conjugate base are approximately equal.
- pKa of the Weak Acid: The buffer is most effective when the pKa of the weak acid is close to the desired pH.
- Temperature: Temperature changes can slightly affect the buffer capacity.
Comparing Acetic Acid/Sodium Acetate with Other Buffer Systems
While the acetic acid/sodium acetate buffer is widely used, other buffer systems exist, each with its own advantages and disadvantages. The choice of buffer depends on the specific requirements of the application, including the desired pH range, buffer capacity, and compatibility with other components in the system. Some examples of other common buffer systems include phosphate buffers, Tris buffers, and citrate buffers.
Conclusion: The Importance of Acetic Acid/Sodium Acetate Buffer Solutions
The acetic acid/sodium acetate buffer system is a powerful tool for maintaining a stable pH in various applications. Its ease of preparation, readily available components, and effectiveness within a useful pH range make it a cornerstone of many scientific and industrial processes. Understanding its properties, preparation, and limitations is crucial for anyone working with buffer solutions. By carefully considering the factors affecting buffer capacity and choosing the appropriate buffer system for a given application, researchers and scientists can ensure the success and reliability of their work. Further exploration into the diverse applications and modifications of this system would reveal its continued significance in advancing various fields. The understanding of buffer chemistry, particularly with the acetic acid/sodium acetate system, continues to be critical for advancements in numerous scientific and industrial fields.
Latest Posts
Latest Posts
-
Is The Flu Virus Lytic Or Lysogenic
Apr 02, 2025
-
Is As A Metal Nonmetal Or Metalloid
Apr 02, 2025
-
The Process Of Getting Information Into Memory Is Called
Apr 02, 2025
-
Where Are Nonmetals Located In The Periodic Table
Apr 02, 2025
-
Write An Equation Any Form For The Quadratic Graphed Below
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Buffer Solution Of Acetic Acid And Sodium Acetate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
