Calculate Average Atomic Mass Of Isotopes
Muz Play
Mar 25, 2025 · 5 min read
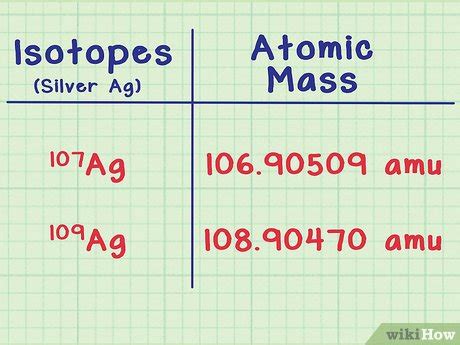
Table of Contents
Calculating the Average Atomic Mass of Isotopes: A Comprehensive Guide
The periodic table presents us with atomic masses that aren't whole numbers. This isn't a mistake; it's a reflection of the existence of isotopes. Understanding how to calculate the average atomic mass of isotopes is crucial for anyone studying chemistry, and this comprehensive guide will walk you through the process step-by-step, exploring the underlying concepts and providing practical examples.
What are Isotopes?
Atoms of the same element, identified by the same atomic number (number of protons), can have different numbers of neutrons. These different forms of the same element are called isotopes. While they share the same chemical properties due to identical electron configurations, their differing neutron counts lead to variations in their mass. This mass difference is significant in calculations involving atomic mass.
For example, carbon has three naturally occurring isotopes: Carbon-12 (¹²C), Carbon-13 (¹³C), and Carbon-14 (¹⁴C). All three have 6 protons, but they have 6, 7, and 8 neutrons, respectively. This accounts for their differing mass numbers (the sum of protons and neutrons).
Understanding Atomic Mass Units (amu)
The mass of atoms is incredibly tiny. To make measurements more manageable, scientists use atomic mass units (amu). One amu is defined as one-twelfth the mass of a single carbon-12 atom. This provides a convenient standard for comparing the masses of different atoms and isotopes.
Calculating Average Atomic Mass: The Formula
The average atomic mass isn't a simple average of the mass numbers of isotopes. Instead, it's a weighted average that considers both the mass of each isotope and its relative abundance in nature. The formula is:
Average Atomic Mass = Σ (Isotope Mass × Isotope Abundance)
Where:
- Σ represents the sum of all isotopes.
- Isotope Mass is the mass of a specific isotope in amu.
- Isotope Abundance is the fractional abundance of that isotope in nature (often expressed as a percentage, which needs to be converted to a decimal).
Step-by-Step Calculation: A Practical Example
Let's calculate the average atomic mass of boron (B), which has two naturally occurring isotopes: ¹⁰B and ¹¹B.
Step 1: Gather the necessary data:
- ¹⁰B: Isotope mass = 10.01 amu, Isotope abundance = 19.9% (or 0.199)
- ¹¹B: Isotope mass = 11.01 amu, Isotope abundance = 80.1% (or 0.801)
Step 2: Apply the formula:
Average Atomic Mass = (10.01 amu × 0.199) + (11.01 amu × 0.801)
Step 3: Perform the calculations:
Average Atomic Mass = 1.99199 amu + 8.81801 amu
Step 4: Determine the final average atomic mass:
Average Atomic Mass = 10.81 amu
Therefore, the average atomic mass of boron is approximately 10.81 amu. This value aligns with the atomic mass of boron found on the periodic table.
Handling Multiple Isotopes
The principle remains the same when dealing with elements possessing more than two isotopes. Simply extend the formula to include all isotopes and their respective masses and abundances.
For instance, consider an element with three isotopes:
- Isotope 1: Mass = M1, Abundance = A1
- Isotope 2: Mass = M2, Abundance = A2
- Isotope 3: Mass = M3, Abundance = A3
The average atomic mass would be calculated as:
Average Atomic Mass = (M1 × A1) + (M2 × A2) + (M3 × A3)
Accuracy and Significance
The accuracy of the calculated average atomic mass depends heavily on the accuracy of the input data – the isotopic masses and abundances. These values are typically determined through sophisticated mass spectrometry techniques. Small variations in reported abundances can slightly affect the final calculated average atomic mass.
Applications of Average Atomic Mass
The average atomic mass has several crucial applications in chemistry and related fields:
- Stoichiometric Calculations: It's essential for determining the molar mass of compounds, which is used extensively in stoichiometric calculations to convert between mass and moles of substances.
- Nuclear Chemistry: Understanding isotopic abundances is critical in nuclear chemistry, particularly in applications like radioactive dating and nuclear medicine.
- Mass Spectrometry: Average atomic mass calculations are intimately linked to the interpretation of data obtained from mass spectrometry, a powerful analytical technique used to identify and quantify the isotopes present in a sample.
Beyond the Basics: Isotopic Enrichment
In some cases, the natural isotopic abundances are altered artificially through a process called isotopic enrichment. This process can increase the concentration of a specific isotope in a sample, leading to a different average atomic mass than what's found naturally. Isotopic enrichment has applications in various fields, including nuclear power generation and medical imaging.
Common Mistakes to Avoid
When calculating average atomic mass, several common mistakes should be avoided:
- Incorrect Unit Conversion: Always ensure that the isotopic abundances are expressed as decimals (fractions) before applying the formula. Using percentages directly will lead to incorrect results.
- Ignoring Isotope Abundances: Remember that the average atomic mass is a weighted average; simply averaging the masses of isotopes without considering their abundances is wrong.
- Mathematical Errors: Double-check your calculations carefully to avoid simple arithmetic mistakes.
Conclusion
Calculating the average atomic mass of isotopes is a fundamental concept in chemistry. Mastering this calculation is crucial for understanding the nature of elements and their behavior in chemical reactions. By carefully following the steps outlined above and understanding the underlying principles, you can confidently calculate the average atomic mass of any element given the necessary data on its isotopes and their abundances. Remember to always double-check your work and be mindful of the potential sources of error. With practice, you'll become proficient in this important calculation and gain a deeper appreciation for the intricacies of atomic structure and the composition of matter.
Latest Posts
Latest Posts
-
On The Weak Acid Strong Base Titration Curve
Mar 28, 2025
-
Label The Cranial Dura Septa In The Figure
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Calculate Average Atomic Mass Of Isotopes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
