Calculate The Degree Of Unsaturation For The Following Molecule
Muz Play
Mar 28, 2025 · 4 min read
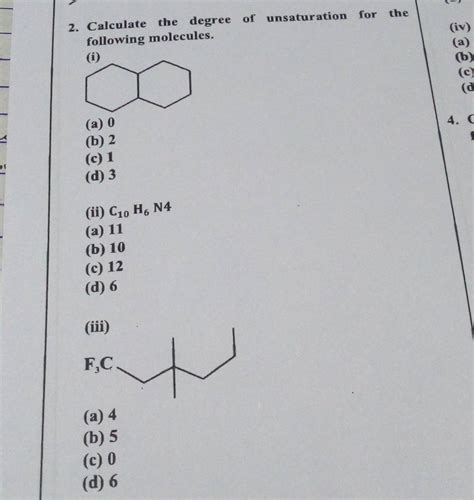
Table of Contents
Calculating the Degree of Unsaturation: A Comprehensive Guide
Determining the degree of unsaturation (also known as the index of hydrogen deficiency or IHD) is a crucial skill in organic chemistry. It allows us to predict the number of rings and/or pi bonds present in a molecule, significantly narrowing down the possibilities when determining its structure. This comprehensive guide will walk you through the process, explaining the underlying principles and providing practical examples. We'll delve deep into the calculation, exploring different scenarios and addressing common misconceptions.
Understanding the Degree of Unsaturation
The degree of unsaturation reflects the number of hydrogen atoms that would need to be added to a molecule to make it fully saturated (containing only single bonds and the maximum number of hydrogen atoms). Each degree of unsaturation represents either a ring or a pi bond (e.g., a double or triple bond).
The Formula
The most common formula for calculating the degree of unsaturation is:
DU = (2C + 2 + N - X - H) / 2
Where:
- C represents the number of carbon atoms.
- N represents the number of nitrogen atoms.
- X represents the number of halogen atoms (F, Cl, Br, I).
- H represents the number of hydrogen atoms.
Important Note: Oxygen and sulfur atoms do not affect the degree of unsaturation. They don't change the number of hydrogen atoms required for saturation.
Step-by-Step Calculation with Examples
Let's illustrate the calculation with several examples, progressing from simple to more complex structures.
Example 1: Ethene (C₂H₄)
Ethene, also known as ethylene, is a simple alkene with a double bond. Let's apply the formula:
- C = 2
- N = 0
- X = 0
- H = 4
DU = (2 * 2 + 2 + 0 - 0 - 4) / 2 = 2 / 2 = 1
The degree of unsaturation is 1, indicating one double bond. This aligns perfectly with the structure of ethene.
Example 2: Benzene (C₆H₆)
Benzene is a classic example of an aromatic compound with a ring structure and alternating double bonds.
- C = 6
- N = 0
- X = 0
- H = 6
DU = (2 * 6 + 2 + 0 - 0 - 6) / 2 = 8 / 2 = 4
The degree of unsaturation is 4. This accounts for the three double bonds and the ring in the benzene structure.
Example 3: Cyclohexane (C₆H₁₂)
Cyclohexane is a saturated cyclic hydrocarbon.
- C = 6
- N = 0
- X = 0
- H = 12
DU = (2 * 6 + 2 + 0 - 0 - 12) / 2 = 2 / 2 = 1
The degree of unsaturation is 1, representing the single ring.
Example 4: A More Complex Molecule: C₈H₁₀N₂O₂
This example introduces heteroatoms (N and O) to demonstrate how to handle them using the formula. Remember, oxygen atoms are ignored.
- C = 8
- N = 2
- X = 0
- H = 10
DU = (2 * 8 + 2 + 2 - 0 - 10) / 2 = 10 / 2 = 5
The molecule has a degree of unsaturation of 5. This could represent a combination of rings and pi bonds. Further analysis would be needed to determine the exact structure.
Example 5: Including Halogens: C₄H₇Cl
This example shows how to handle halogens. Remember, halogens are treated as if they were hydrogens.
- C = 4
- N = 0
- X = 1 (Chlorine)
- H = 7
DU = (2 * 4 + 2 + 0 - 1 - 7) / 2 = 2 / 2 = 1
This molecule has a degree of unsaturation of 1, which could be either a double bond or a ring.
Interpreting the Degree of Unsaturation
The degree of unsaturation provides valuable information, but it doesn't definitively reveal the structure. For instance, a DU of 2 could represent:
- Two double bonds
- One triple bond
- One ring and one double bond
- Two rings
Additional information is necessary for complete structural elucidation. Techniques such as spectroscopy (NMR, IR, Mass Spectrometry) provide crucial information to determine the arrangement of atoms and functional groups within the molecule.
Common Mistakes and Troubleshooting
- Forgetting to account for heteroatoms: Always remember to include nitrogen and halogens in the calculation. Oxygen and sulfur are ignored.
- Incorrectly applying the formula: Double-check your calculations to ensure you're correctly substituting the values.
- Misinterpreting the result: Remember that the DU only indicates the total number of rings and pi bonds; it doesn't specify their arrangement.
Beyond the Basic Formula
For more complex molecules containing phosphorus, silicon, or other elements, modifications to the formula might be necessary. Consult advanced organic chemistry texts for these less common scenarios.
Conclusion: A Powerful Tool in Structural Elucidation
Calculating the degree of unsaturation is a fundamental skill in organic chemistry. It provides a quick and efficient way to gain initial insights into the possible structures of unknown molecules. Combining this information with other spectroscopic techniques allows for accurate structure determination. Mastering this calculation significantly improves problem-solving capabilities within the field of organic chemistry and related disciplines. Remember to practice with various examples to build confidence and understanding. Through repeated practice and careful consideration of the formula and its implications, you will become proficient in determining the degree of unsaturation and unlocking the structural mysteries of organic molecules.
Latest Posts
Latest Posts
-
Truth Table With P Then Q
Mar 31, 2025
-
Gas In A Gas Solution Example
Mar 31, 2025
-
Which Of The Following Cross Couplings Of An Enolate
Mar 31, 2025
-
Primary And Secondary Growth Of Plants
Mar 31, 2025
-
Relationship Between Temperature And Kinetic Energy
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Degree Of Unsaturation For The Following Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
