Calculate The Ph Of A Weak Base
Muz Play
Mar 16, 2025 · 6 min read
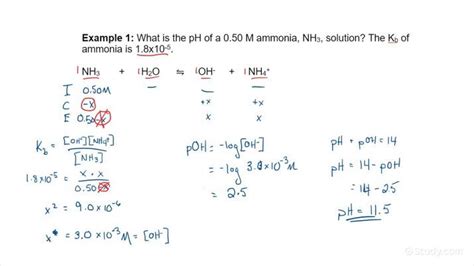
Table of Contents
Calculating the pH of a Weak Base: A Comprehensive Guide
Calculating the pH of a weak base solution might seem daunting at first, but with a systematic approach and understanding of the underlying chemistry, it becomes manageable. This comprehensive guide will walk you through the process, explaining the concepts, providing step-by-step calculations, and offering helpful tips for success. We'll cover various scenarios, including calculations involving Kb, initial concentrations, and the effects of dilution.
Understanding Weak Bases and Their Behavior in Water
Unlike strong bases, which completely dissociate in water, weak bases only partially ionize. This means that only a small fraction of the base molecules react with water to form hydroxide ions (OH⁻) and their conjugate acid. The extent of ionization is quantified by the base dissociation constant (Kb). A smaller Kb value indicates a weaker base, meaning less ionization and a lower concentration of OH⁻ ions.
The general reaction for a weak base, B, in water is:
B(aq) + H₂O(l) ⇌ BH⁺(aq) + OH⁻(aq)
The equilibrium expression for this reaction is:
Kb = [BH⁺][OH⁻] / [B]
Where:
- [BH⁺] represents the concentration of the conjugate acid.
- [OH⁻] represents the concentration of hydroxide ions.
- [B] represents the concentration of the weak base.
This Kb value is crucial for calculating the pH of a weak base solution.
Calculating pH Using the Kb Value and ICE Table
The most common method for calculating the pH of a weak base solution involves using an ICE (Initial, Change, Equilibrium) table. This table helps organize the concentrations of reactants and products at different stages of the reaction. Let's illustrate this with an example.
Example: Calculate the pH of a 0.10 M solution of ammonia (NH₃), a weak base with Kb = 1.8 x 10⁻⁵.
Step 1: Write the equilibrium reaction and the Kb expression.
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
Kb = [NH₄⁺][OH⁻] / [NH₃] = 1.8 x 10⁻⁵
Step 2: Construct the ICE table.
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| NH₃ | 0.10 | -x | 0.10 - x |
| NH₄⁺ | 0 | +x | x |
| OH⁻ | 0 | +x | x |
Step 3: Substitute the equilibrium concentrations into the Kb expression.
1.8 x 10⁻⁵ = (x)(x) / (0.10 - x)
Step 4: Solve for x. Since Kb is small, we can often approximate 0.10 - x ≈ 0.10, simplifying the equation:
1.8 x 10⁻⁵ = x² / 0.10
x² = 1.8 x 10⁻⁶
x = [OH⁻] = 1.34 x 10⁻³ M
Step 5: Calculate the pOH.
pOH = -log[OH⁻] = -log(1.34 x 10⁻³) ≈ 2.87
Step 6: Calculate the pH.
pH + pOH = 14
pH = 14 - pOH = 14 - 2.87 ≈ 11.13
Therefore, the pH of a 0.10 M solution of ammonia is approximately 11.13.
Addressing the Approximation: When is it Valid?
The approximation (0.10 - x ≈ 0.10) is valid when x is significantly smaller than the initial concentration of the weak base (typically less than 5%). If this condition is not met, you must solve the quadratic equation directly. This involves using the quadratic formula:
x = [-b ± √(b² - 4ac)] / 2a
Where the equation is rearranged to the form ax² + bx + c = 0. While more complex, this approach ensures a more accurate result.
Calculating pH with Different Initial Concentrations
The process remains the same regardless of the initial concentration of the weak base. However, remember that the approximation (0.10 - x ≈ 0.10) becomes less valid as the initial concentration decreases or as the Kb value increases. Always check the 5% rule to determine if the approximation is justified. If not, use the quadratic formula for precise results.
The Impact of Dilution on pH
Diluting a weak base solution will decrease the concentration of hydroxide ions, leading to a decrease in pOH and an increase in pH. However, the change in pH is not directly proportional to the dilution factor, as the equilibrium shifts to partially compensate for the dilution. The ICE table method, along with the appropriate approximation or quadratic solution, allows you to accurately calculate the pH after dilution.
Dealing with Polyprotic Weak Bases
Polyprotic weak bases can donate more than one proton. Calculating the pH becomes more complex as it often requires considering multiple equilibrium reactions and their respective Kb values. In many cases, the first ionization step significantly dominates the pH, and you can approximate the pH based on the first Kb value only. However, for greater accuracy, especially if the Kb values are similar in magnitude, it’s crucial to account for all ionization steps.
Utilizing pKb and its Relationship to pH
The pKb is the negative logarithm of the Kb value: pKb = -log(Kb). It's a convenient way to express the base strength. The relationship between pKb and pH is indirect but related through the pOH and the equation:
pKw = pH + pOH = 14 (at 25°C)
You can determine pOH from pKb and use this relationship to find the pH.
Practical Applications and Significance
Understanding how to calculate the pH of weak bases is crucial in various fields:
- Environmental Chemistry: Determining the pH of natural water bodies, assessing the impact of pollutants, and monitoring water quality.
- Biochemistry: Understanding the pH of biological systems and the behavior of weak bases in living organisms.
- Analytical Chemistry: Titrations, buffer solutions, and other analytical techniques rely on understanding weak base behavior.
- Pharmaceutical Chemistry: Many drugs are weak bases, and knowing their pH is vital for formulation and delivery.
Advanced Scenarios and Considerations
This guide has covered the fundamental principles. More complex scenarios may involve:
- Common Ion Effect: The presence of a common ion (e.g., adding a salt containing the conjugate acid) can significantly affect the pH of a weak base solution.
- Temperature Dependence: Kb values are temperature-dependent, and calculations may need adjustment for temperatures significantly different from 25°C.
- Activity Coefficients: At higher concentrations, deviations from ideal behavior can occur, necessitating the use of activity coefficients to correct for intermolecular interactions.
Mastering the basic methods outlined here is a crucial step towards understanding these more complex scenarios.
Conclusion: Mastering pH Calculations for Weak Bases
Calculating the pH of a weak base solution is a fundamental skill in chemistry. While the basic principle remains constant, the complexity increases with factors such as polyprotic bases, varying initial concentrations, and considerations for precise calculations. By understanding the use of ICE tables, approximations, the quadratic formula, and the significance of Kb and pKb, you gain a strong foundation to approach pH calculations with confidence. Remember to always check the validity of your approximations and consider more advanced techniques when needed to ensure accurate and meaningful results. This detailed approach will assist you in tackling various scenarios and applying this knowledge effectively in different contexts.
Latest Posts
Latest Posts
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
-
A Temporary Mixture The Particles Will Eventually Settle
Mar 17, 2025
-
Why Do Ions Travel Back And Forth In Orbitrap
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Ph Of A Weak Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
