Calculating An Equilibrium Constant From A Partial Equilibrium Composition
Muz Play
Mar 24, 2025 · 5 min read
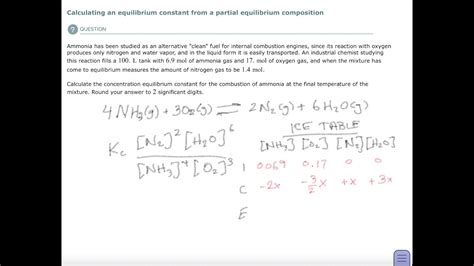
Table of Contents
Calculating an Equilibrium Constant from a Partial Equilibrium Composition
Determining the equilibrium constant (K) is crucial in chemistry for understanding the extent of a reaction and predicting its direction under various conditions. While direct measurement of all equilibrium concentrations is ideal, it's not always feasible. This article delves into the practical approach of calculating K from a partial equilibrium composition, a scenario frequently encountered in experimental settings. We will explore the underlying principles, the necessary data, common calculation methods, and the potential sources of error.
Understanding Equilibrium Constants and Partial Equilibrium
The equilibrium constant, K, represents the ratio of products to reactants at equilibrium, each raised to the power of its stoichiometric coefficient. For a generic reversible reaction:
aA + bB ⇌ cC + dD
The equilibrium constant expression is:
K = ([C]^c [D]^d) / ([A]^a [B]^b)
Where [A], [B], [C], and [D] represent the equilibrium concentrations of the respective species. A large K value signifies that the equilibrium favors product formation, while a small K value indicates reactant dominance.
Partial equilibrium arises when the system reaches equilibrium with respect to some, but not all, of the reaction components. This can occur due to several reasons:
- Slow reactions: Some reactions proceed very slowly, preventing the complete attainment of equilibrium within a reasonable timeframe.
- Intermediate species: Complex reactions involving multiple steps may reach equilibrium for some intermediate steps before the overall reaction reaches completion.
- External factors: External interventions, such as the continuous addition or removal of reactants or products, can disrupt the overall equilibrium while maintaining a partial equilibrium state for certain components.
Data Required for Calculating K from Partial Equilibrium
Accurately calculating K from partial equilibrium requires specific experimental data. This typically includes:
- Initial concentrations: The concentrations of all reactants and products at the start of the reaction are essential.
- Equilibrium concentrations of some species: While you might not know the equilibrium concentration of all species, you need the equilibrium concentrations of at least one reactant and one product, or a sufficient number of species to solve for the unknowns.
- Stoichiometry of the reaction: The balanced chemical equation provides the crucial stoichiometric coefficients used in the equilibrium constant expression.
- Temperature: The equilibrium constant is temperature-dependent; therefore, the temperature at which the measurements are taken must be recorded.
Methods for Calculating K from Partial Equilibrium
The specific method for calculating K depends on the available data. Let's explore a few common scenarios:
1. Using ICE Tables (Initial, Change, Equilibrium)
ICE tables provide a structured approach, especially helpful when dealing with simpler reactions where changes in concentration can be easily determined.
Example: Consider the reaction: N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
Let's assume we know the initial concentrations of N₂ and H₂, and the equilibrium concentration of NH₃. We can represent this in an ICE table:
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| N₂ | [N₂]₀ | -x | [N₂]₀ - x |
| H₂ | [H₂]₀ | -3x | [H₂]₀ - 3x |
| NH₃ | 0 | +2x | 2x |
We know the equilibrium concentration of NH₃ (2x). Solving for x, we can then determine the equilibrium concentrations of N₂ and H₂ ([N₂]₀ - x and [H₂]₀ - 3x respectively). Finally, we can substitute these values into the equilibrium constant expression:
K = ([NH₃]²) / ([N₂][H₂]³)
2. Utilizing Spectroscopic Techniques
Spectroscopic methods like UV-Vis, IR, or NMR spectroscopy can directly measure the concentrations of certain species in a mixture. This data can then be used to calculate K, even if not all species are directly measured.
For instance, if we can determine the equilibrium concentration of one reactant and one product using spectroscopy, we can still utilize the stoichiometry of the reaction to calculate the equilibrium concentrations of the other components using the ICE table method or similar techniques.
3. Employing Advanced Equilibrium Calculations with Multiple Equilibria
In complex systems with multiple simultaneous equilibria, solving for K requires more sophisticated techniques, often involving solving simultaneous equations. This frequently involves using iterative numerical methods and computer software to arrive at the solution.
Potential Sources of Error and Limitations
Calculating K from partial equilibrium composition presents certain challenges and potential errors:
- Incomplete Equilibrium: The primary limitation stems from the fact that the system might not have fully reached equilibrium. This introduces significant inaccuracies in the calculated K value.
- Experimental Errors: Errors in measuring initial and equilibrium concentrations can lead to substantial errors in the calculated K. This includes errors in instrumental readings, sampling techniques, and sample preparation.
- Side Reactions: The presence of undesired side reactions can consume reactants or products, thus affecting the equilibrium composition and the K calculation.
- Non-Ideal Behavior: The calculation of K assumes ideal behavior of the system. Deviations from ideality, particularly at higher concentrations, introduce errors in the calculations. Activity coefficients might be needed to correct for non-ideal behavior.
- Temperature Fluctuations: Changes in temperature throughout the experiment can affect equilibrium compositions, thereby impacting the accuracy of K. Maintaining constant temperature is crucial.
Improving the Accuracy of K Calculation
To enhance the accuracy of the equilibrium constant calculation from partial equilibrium data:
- Accurate Measurements: Employ highly precise measurement techniques to minimize experimental errors.
- Multiple Measurements: Perform multiple experiments to obtain multiple data sets, which can then be averaged to reduce the impact of random errors.
- Error Analysis: Perform a detailed error analysis to quantify the uncertainty associated with the calculated K value.
- Advanced Techniques: Utilize advanced analytical techniques to accurately determine the concentration of multiple components.
- Control of External Factors: Careful control of temperature, pressure, and other external factors can minimize their impact on equilibrium.
- Consider Non-Ideal Behavior: If necessary, incorporate activity coefficients to account for non-ideal behavior.
Conclusion
Calculating the equilibrium constant from partial equilibrium composition requires careful consideration of various factors. While not as straightforward as measuring all equilibrium concentrations directly, it's a practical approach with wide applications in chemical analysis and reaction engineering. Careful experimental design, accurate measurements, and appropriate data analysis techniques are crucial for obtaining a reliable estimate of the equilibrium constant. The choice of calculation method depends heavily on the type and amount of available data, ranging from simple ICE tables to sophisticated numerical methods for complex systems. Always be aware of the potential sources of error and take steps to minimize their impact. Understanding these limitations allows for a more accurate interpretation of the calculated K value and its implications.
Latest Posts
Latest Posts
-
Do Homologous Chromosomes Pair In Mitosis
Mar 27, 2025
-
How To Find Concentration In Titration
Mar 27, 2025
-
In A Uniform Circular Motion What Is Constant
Mar 27, 2025
-
Homologous Chromosomes Line Up In The Center Of The Cell
Mar 27, 2025
-
Where Is This Energy Stored In Glucose
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Calculating An Equilibrium Constant From A Partial Equilibrium Composition . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
