How To Find Concentration In Titration
Muz Play
Mar 27, 2025 · 6 min read
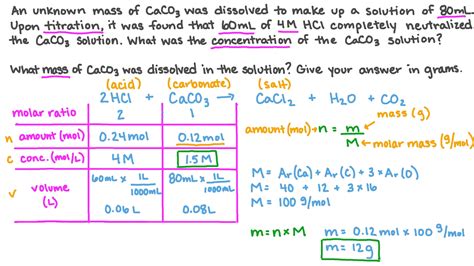
Table of Contents
How to Find Concentration in Titration: A Comprehensive Guide
Titration, a fundamental technique in analytical chemistry, allows us to determine the unknown concentration of a substance by reacting it with a solution of known concentration. Mastering titration involves meticulous technique and a solid understanding of stoichiometry. This comprehensive guide will delve into the intricacies of finding concentration in titration, covering everything from preparation to calculation, ensuring you achieve accurate and reliable results.
Understanding the Fundamentals of Titration
Before diving into the practical aspects, let's solidify our understanding of the underlying principles. Titration relies on a quantitative chemical reaction between two solutions: the analyte (solution with unknown concentration) and the titrant (solution with known concentration). The reaction is typically an acid-base neutralization, a redox reaction, or a precipitation reaction.
Key Terms and Concepts:
- Analyte: The solution whose concentration we want to determine.
- Titrant: The solution of known concentration added to the analyte.
- Equivalence point: The point in the titration where the moles of titrant added are stoichiometrically equivalent to the moles of analyte.
- Endpoint: The point in the titration where a noticeable color change (using an indicator) or a significant change in pH (using a pH meter) occurs. Ideally, the endpoint should be very close to the equivalence point.
- Indicator: A substance that changes color near the equivalence point, providing a visual signal of the reaction's completion. The choice of indicator depends on the pH range of the equivalence point.
- Molarity (M): The concentration of a solution expressed as moles of solute per liter of solution.
Preparing for a Successful Titration
Accurate results hinge on careful preparation. Overlooking even small details can lead to significant errors.
1. Selecting the Appropriate Titrant and Indicator:
The choice of titrant depends on the nature of the analyte. For instance:
- Acid-base titrations: Strong acids (e.g., HCl, H₂SO₄) are often titrated with strong bases (e.g., NaOH, KOH), and vice versa. The indicator should change color near the equivalence point's pH. Phenolphthalein (pH range 8.2-10.0) is a common choice for strong acid-strong base titrations.
- Redox titrations: Potassium permanganate (KMnO₄) and potassium dichromate (K₂Cr₂O₇) are frequently used as titrants. The endpoint is often visually determined by the color change of the titrant itself or a redox indicator.
- Complexometric titrations: EDTA (ethylenediaminetetraacetic acid) is a common titrant used to determine the concentration of metal ions.
2. Preparing the Solutions:
Accurate preparation of solutions is crucial:
- Standard solutions: The titrant must be a standard solution, meaning its concentration is precisely known. This is often achieved through careful weighing and dilution of a primary standard (a highly pure substance).
- Analyte solution: The concentration of the analyte solution should be appropriately chosen to ensure the titration volume is within a reasonable range (typically 20-30 mL). Diluting the analyte if necessary is recommended.
3. Cleaning and Preparing Glassware:
Cleanliness is paramount:
- Burette: Rinse thoroughly with distilled water, followed by a small amount of the titrant, to eliminate any contamination.
- Pipette: Rinse thoroughly with distilled water, followed by the analyte solution, before pipetting the desired volume of analyte into the Erlenmeyer flask.
- Erlenmeyer flask: Rinse with distilled water to ensure no interfering substances remain.
4. Setting up the Apparatus:
Proper setup minimizes errors:
- Burette clamp: Securely clamp the burette to a retort stand, ensuring it is vertical.
- Filling the burette: Carefully fill the burette with the titrant, ensuring no air bubbles remain in the burette tip. Record the initial burette reading accurately.
Performing the Titration
Careful technique during the titration is essential for accuracy:
1. Adding the Analyte:
Pipette a precise volume of the analyte solution into a clean Erlenmeyer flask. Add a few drops of the appropriate indicator.
2. Adding the Titrant:
Initially, add the titrant rapidly, swirling the flask constantly to ensure thorough mixing. As the endpoint approaches, slow the addition to a drop-wise manner, swirling continuously. Observe the color change carefully.
3. Reaching the Endpoint:
The endpoint is reached when a persistent color change is observed, signifying the completion of the reaction. Record the final burette reading precisely.
4. Repeating the Titration:
It is best practice to repeat the titration at least two or three times. The results should be consistent within a reasonable range.
Calculations and Data Analysis
After completing the titration, the next step is to calculate the unknown concentration.
1. Calculating the Volume of Titrant Used:
Subtract the initial burette reading from the final burette reading to determine the volume of titrant used.
2. Calculating the Moles of Titrant Used:
Use the following formula to calculate the moles of titrant used:
Moles of titrant = Molarity of titrant (M) × Volume of titrant (L)
3. Using Stoichiometry to Calculate Moles of Analyte:
The stoichiometric ratio between the titrant and analyte, based on the balanced chemical equation, determines the moles of analyte. For example, in a 1:1 reaction:
Moles of analyte = Moles of titrant
For reactions with different stoichiometric ratios, adjust accordingly.
4. Calculating the Concentration of Analyte:
Finally, calculate the concentration of the analyte using the following formula:
Molarity of analyte (M) = Moles of analyte / Volume of analyte (L)
5. Statistical Analysis:
For multiple titrations, calculate the average concentration and standard deviation to evaluate precision and accuracy. Outliers should be investigated.
Common Sources of Error in Titration
Awareness of potential errors enhances accuracy:
- Improperly calibrated glassware: Using inaccurate burettes or pipettes leads to significant errors.
- Incorrect indicator choice: An indicator that changes color far from the equivalence point results in inaccurate results.
- Incomplete mixing: Insufficient swirling during titration prevents complete reaction.
- Air bubbles in the burette: Air bubbles in the burette tip lead to an inaccurate volume measurement.
- Parallax error: Improper reading of the meniscus in the burette.
- Contamination: Contamination of glassware or solutions can interfere with the reaction.
- Improper endpoint detection: Misjudging the endpoint leads to inaccurate readings.
Advanced Techniques and Considerations
Beyond basic titrations, several advanced techniques improve precision and accuracy:
- pH meter: Using a pH meter instead of an indicator allows for precise determination of the equivalence point, particularly in weak acid-weak base titrations.
- Potentiometric titration: This technique involves monitoring the potential difference between two electrodes during the titration to determine the equivalence point.
- Automatic titrators: Automated titrators enhance precision, speed, and reduce human error.
Conclusion
Mastering titration requires careful preparation, meticulous technique, and a thorough understanding of stoichiometry. By following the steps outlined in this guide, you can achieve accurate and reliable results in determining the concentration of unknown solutions. Remember to always practice safe laboratory techniques and handle chemicals appropriately. The principles of titration are fundamental to many analytical procedures and a solid grasp of them is essential for anyone working in analytical chemistry or related fields. Continuous practice and attention to detail are key to mastering this invaluable analytical skill. By consistently applying these methods and being mindful of potential sources of error, you can ensure your titration results are both precise and accurate.
Latest Posts
Latest Posts
-
Is Density And Specific Gravity The Same
Mar 30, 2025
-
Which Complex Carbohydrate Contains Only A 1 4 Glycosidic Linkages
Mar 30, 2025
-
Understanding Media And Culture An Introduction To Mass Communication
Mar 30, 2025
-
The Substance That Is Being Dissolved By A Solvent
Mar 30, 2025
-
What Percentage Of Alcohol Is Absorbed Through The Small Intestine
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How To Find Concentration In Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
