Which Complex Carbohydrate Contains Only A 1 4 Glycosidic Linkages
Muz Play
Mar 30, 2025 · 5 min read
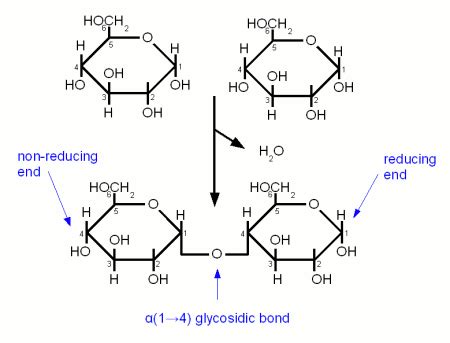
Table of Contents
Which Complex Carbohydrate Contains Only 1,4 Glycosidic Linkages? Exploring the Structure and Function of Starch
The world of carbohydrates is vast and complex, encompassing a wide array of molecules crucial for life. While simple sugars like glucose and fructose are readily recognizable, the realm of complex carbohydrates presents a greater challenge in understanding their diverse structures and functions. One key aspect differentiating these complex structures lies in the type of glycosidic linkages holding their monosaccharide units together. This article delves deep into the question: which complex carbohydrate contains only 1,4 glycosidic linkages? While the answer isn't a straightforward single molecule, we'll explore the intricacies of starch, a crucial energy storage polysaccharide that predominantly, but not exclusively, utilizes 1,4 glycosidic bonds.
Understanding Glycosidic Linkages
Before diving into the specifics of starch, it's crucial to understand what glycosidic linkages are. Glycosidic bonds are covalent bonds that link monosaccharides (simple sugars) together to form disaccharides (like sucrose) and polysaccharides (like starch and cellulose). The numbering in "1,4 glycosidic linkage" refers to the carbon atoms involved in the bond formation. The "1" indicates the carbon atom on the first monosaccharide unit, and the "4" refers to the carbon atom on the second monosaccharide unit. The configuration of this bond (α or β) also plays a significant role in the properties of the resulting polysaccharide.
Alpha (α) vs. Beta (β) Glycosidic Linkages
The orientation of the hydroxyl group (-OH) on the carbon atom involved in the glycosidic bond distinguishes between α and β linkages. In α-glycosidic linkages, the hydroxyl group is below the plane of the ring (in the Haworth projection), while in β-glycosidic linkages, it's above the plane. This seemingly minor difference has profound effects on the overall structure and digestibility of polysaccharides.
Starch: A Predominantly 1,4 Glycosidic Linked Polysaccharide
Starch is the primary energy storage polysaccharide in plants. It’s composed of two main components:
-
Amylose: A linear polymer of glucose units linked by α-1,4 glycosidic bonds. This is the key component answering the core question of the article. Amylose forms a helical structure due to these linkages.
-
Amylopectin: A branched polymer of glucose units. While primarily linked by α-1,4 glycosidic bonds, amylopectin also contains α-1,6 glycosidic branches approximately every 24-30 glucose units. These branches create a more compact and less crystalline structure than amylose.
Therefore, while amylose, a major component of starch, does consist solely of α-1,4 glycosidic linkages, starch itself is not solely composed of these linkages due to the presence of amylopectin's α-1,6 branches. This distinction is crucial for understanding the nuances of the question.
The Significance of α-1,4 Glycosidic Linkages in Starch
The prevalence of α-1,4 glycosidic bonds in amylose and the significant portion of amylopectin imparts several critical properties to starch:
1. Energy Storage Efficiency:
The α-1,4 linkages facilitate efficient packing of glucose units into a relatively compact structure. This compact structure maximizes energy storage within plant cells without excessive osmotic pressure.
2. Enzymatic Degradation:
The α-configuration of the glycosidic bonds allows for easy enzymatic breakdown by human digestive enzymes (amylase). Amylase specifically targets α-1,4 linkages, releasing glucose units for energy production. This ease of digestion contrasts sharply with cellulose, which contains β-1,4 linkages and is indigestible by humans.
3. Water Solubility and Gelatinization:
The α-1,4 linkages, particularly in amylose, contribute to the water solubility and gelatinization properties of starch. When heated in water, starch granules swell and eventually burst, forming a viscous gel. This property is crucial in many food applications.
4. Crystalline Structure:
The regular arrangement of glucose units linked by α-1,4 glycosidic bonds in amylose contributes to its relatively crystalline structure. This crystallinity influences the physical properties of starch, such as its texture and digestibility.
Other Polysaccharides and Their Glycosidic Linkages
To fully appreciate the uniqueness of starch's predominantly 1,4 α-glycosidic structure, let's compare it with other important polysaccharides:
Cellulose:
Cellulose, a major structural component of plant cell walls, is a polymer of glucose units linked by β-1,4 glycosidic bonds. This subtle difference in linkage configuration has a massive impact on its properties. β-1,4 linkages create a linear, rigid structure, forming strong microfibrils resistant to degradation by human enzymes.
Glycogen:
Glycogen, the primary energy storage polysaccharide in animals, is structurally similar to amylopectin. It's a highly branched polymer of glucose units linked by α-1,4 glycosidic bonds with α-1,6 branches. However, glycogen has more frequent branching than amylopectin, resulting in a more compact and readily accessible energy store.
Chitin:
Chitin is a structural polysaccharide found in the exoskeletons of insects and crustaceans. It's a polymer of N-acetylglucosamine units linked by β-1,4 glycosidic bonds, similar to cellulose but with a modified monosaccharide unit.
The Importance of Starch in the Diet
Starch constitutes a significant portion of the human diet, providing a crucial source of energy. The readily digestible nature of its α-1,4 linkages ensures efficient glucose release, fueling metabolic processes. However, excessive starch intake can lead to various health issues, highlighting the need for a balanced diet.
Industrial Applications of Starch
Beyond its dietary importance, starch finds extensive application in various industries:
- Food industry: As a thickening agent, stabilizer, and gelling agent in numerous food products.
- Textile industry: As a sizing agent for fabrics.
- Paper industry: As a binder and coating material.
- Pharmaceutical industry: As a binder and disintegrant in tablets and capsules.
Conclusion: Amylose, the 1,4-Linked Hero
To reiterate, while starch itself contains both α-1,4 and α-1,6 glycosidic linkages, amylose, a significant component of starch, contains only α-1,4 glycosidic linkages. This specific structure is crucial for its role in energy storage, digestibility, and various industrial applications. Understanding the intricacies of glycosidic linkages and their impact on the properties of polysaccharides is essential for appreciating the complexity and importance of these biomolecules in biological systems and various industries. Further research continues to unravel the precise mechanisms of starch synthesis, degradation, and its impact on human health and industrial processes. The subtle differences in glycosidic linkages have far-reaching consequences, underscoring the importance of studying these fundamental molecular structures.
Latest Posts
Latest Posts
-
How To Tell If A Function Is Continuous Without Graphing
Apr 01, 2025
-
How To Shift A Function To The Right
Apr 01, 2025
-
What Is The Second Step Of Photosynthesis
Apr 01, 2025
-
Is A Base A Proton Acceptor
Apr 01, 2025
-
Area Of A Parallelogram Cross Product
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Complex Carbohydrate Contains Only A 1 4 Glycosidic Linkages . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
