Is A Base A Proton Acceptor
Muz Play
Apr 01, 2025 · 5 min read
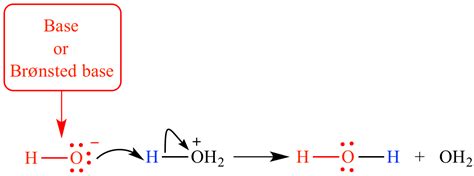
Table of Contents
Is a Base a Proton Acceptor? A Deep Dive into Acid-Base Chemistry
The question, "Is a base a proton acceptor?" is fundamental to understanding acid-base chemistry. The short answer is a resounding yes, but the nuances of this definition require a deeper exploration. This article will delve into the various acid-base theories, exploring the concept of proton acceptance in detail and examining its implications for chemical reactions and properties.
Brønsted-Lowry Acid-Base Theory: The Cornerstone of Proton Transfer
The most common definition of a base hinges on the Brønsted-Lowry theory. This theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, defines an acid as a proton (H⁺) donor and a base as a proton acceptor. This definition elegantly explains a vast array of acid-base reactions, focusing on the transfer of a proton from the acid to the base.
Understanding Proton Transfer
The key to understanding Brønsted-Lowry theory lies in visualizing the proton transfer process. When an acid reacts with a base, the acid donates a proton to the base. This proton, essentially a hydrogen ion (H⁺), is highly reactive due to its positive charge and lack of electrons. The base, with its lone pair of electrons or negatively charged atom, readily accepts this proton, forming a new bond.
Example: Consider the reaction between hydrochloric acid (HCl) and water (H₂O):
HCl + H₂O ⇌ H₃O⁺ + Cl⁻
In this reaction, HCl acts as the acid, donating a proton to H₂O, which acts as the base. The result is the formation of the hydronium ion (H₃O⁺) and the chloride ion (Cl⁻). Notice the proton (H⁺) has moved from the HCl to the H₂O.
Conjugate Acid-Base Pairs
The Brønsted-Lowry theory also introduces the concept of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. In the HCl and H₂O reaction above:
- HCl (acid) and Cl⁻ (conjugate base) form a conjugate pair.
- H₂O (base) and H₃O⁺ (conjugate acid) form a conjugate pair.
This concept highlights the reversible nature of many acid-base reactions. The conjugate acid can donate a proton back to the conjugate base, reforming the original acid and base.
Expanding the Definition: Beyond Proton Acceptance
While the Brønsted-Lowry definition is incredibly useful, it's important to acknowledge that not all bases function solely by accepting protons. Other acid-base theories provide alternative perspectives.
Lewis Acid-Base Theory: A Broader Perspective
The Lewis acid-base theory, proposed by Gilbert N. Lewis, offers a more generalized definition. A Lewis acid is defined as an electron-pair acceptor, and a Lewis base is an electron-pair donor. This definition encompasses a wider range of reactions than the Brønsted-Lowry theory.
The Role of Electron Pairs
The Lewis theory focuses on the movement of electron pairs rather than just protons. A Lewis base, possessing a lone pair of electrons, donates this pair to a Lewis acid, which has an empty orbital capable of accepting the electron pair. This leads to the formation of a coordinate covalent bond (also known as a dative bond).
Bridging the Theories
While seemingly different, the Brønsted-Lowry and Lewis theories are interconnected. Many Brønsted-Lowry bases are also Lewis bases. For example, the hydroxide ion (OH⁻) acts as a Brønsted-Lowry base by accepting a proton, and it also acts as a Lewis base by donating its lone pair of electrons.
However, not all Lewis bases are Brønsted-Lowry bases. Some molecules, such as ammonia (NH₃), can donate their lone pair to form a coordinate covalent bond without directly accepting a proton. This illustrates the broader scope of the Lewis definition.
Amphoteric Substances: Acting as Both Acid and Base
Certain substances can act as both acids and bases, depending on the reaction conditions. These are known as amphoteric substances. Water is a classic example:
- As a base: H₂O + HCl ⇌ H₃O⁺ + Cl⁻ (accepts a proton)
- As an acid: H₂O + NH₃ ⇌ NH₄⁺ + OH⁻ (donates a proton)
The ability to act as both an acid and a base depends on the relative strengths of the acid and base involved in the reaction.
The Importance of Solvent Considerations
The strength of an acid or base is not an absolute property; it's highly dependent on the solvent. A weak acid in one solvent might behave as a strong acid in another. This is because the solvent can influence the equilibrium of the proton transfer reaction.
Example: Acetic acid (CH₃COOH) is a weak acid in water, but it behaves as a stronger acid in a less polar solvent like ethanol. This highlights the interplay between the solvent and the acid-base reaction.
Practical Applications of Proton Acceptance
Understanding proton acceptance is crucial in various fields:
- Analytical Chemistry: Acid-base titrations rely on the quantitative transfer of protons between acids and bases.
- Biochemistry: Many biological processes involve proton transfer, including enzyme catalysis and protein folding. The pH of biological systems is meticulously controlled to maintain proper function. Many biological molecules act as buffers, resisting changes in pH by accepting or donating protons.
- Industrial Chemistry: Acid-base reactions are essential in various industrial processes, such as the production of fertilizers, pharmaceuticals, and polymers.
- Environmental Chemistry: Understanding acid-base chemistry is crucial for managing environmental issues like acid rain and water pollution.
Conclusion: The Centrality of Proton Acceptance
In conclusion, while the simple answer to "Is a base a proton acceptor?" is definitively yes, the topic extends far beyond this simple statement. The Brønsted-Lowry theory provides a clear and widely applicable definition of bases as proton acceptors, highlighting the importance of proton transfer in acid-base reactions and the concept of conjugate acid-base pairs. However, the Lewis theory expands our understanding by emphasizing electron pair donation, encompassing a broader range of acid-base reactions. The interplay between solvent effects and the amphoteric nature of certain substances further complicates—and enriches—our understanding of this fundamental aspect of chemistry. The concept of proton acceptance remains central to understanding chemical reactivity and is indispensable across many scientific and industrial disciplines. Further exploration into the specific characteristics of different bases and their interactions with various acids unveils the rich complexity of acid-base chemistry.
Latest Posts
Latest Posts
-
What Would The Potential Of A Standard Hydrogen Electrode
Apr 02, 2025
-
Draw The Tautomer Of This Aldehyde
Apr 02, 2025
-
What Is A Non Rigid Transformation
Apr 02, 2025
-
Write The Equation In Spherical Coordinates
Apr 02, 2025
-
In What Form Do Fats First Enter The Bloodstream
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is A Base A Proton Acceptor . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
