Where Is This Energy Stored In Glucose
Muz Play
Mar 27, 2025 · 6 min read
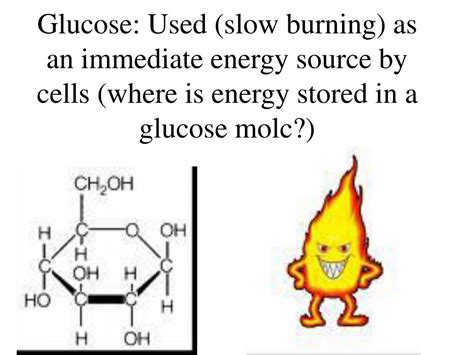
Table of Contents
Where is This Energy Stored in Glucose? Unpacking the Secrets of Cellular Fuel
Glucose, the simple sugar that fuels our cells, isn't simply a source of immediate energy. Its power lies in the intricate arrangement of its atoms and the potential energy locked within its chemical bonds. Understanding where this energy is stored requires delving into the molecular structure of glucose and the biochemical processes that release it. This article will explore the fascinating world of glucose energy storage, from its chemical bonds to the cellular machinery that harnesses its power.
The Molecular Structure: A Blueprint of Energy
Glucose, a six-carbon monosaccharide (C₆H₁₂O₆), exists in several isomeric forms, but the most biologically relevant is D-glucose. Its structure is crucial to understanding its energy storage. The molecule isn't simply a linear chain of carbon, hydrogen, and oxygen atoms; it's a cyclic structure with several key features:
1. Carbon-Carbon Bonds:
The backbone of the glucose molecule consists of a ring of six carbon atoms. The carbon-carbon bonds (C-C) store a significant amount of potential energy. These bonds aren't particularly strong individually, but the cumulative energy stored in all the C-C bonds within a glucose molecule is substantial. Breaking these bonds through cellular respiration is the primary method of releasing this stored energy.
2. Carbon-Hydrogen Bonds:
Numerous carbon-hydrogen bonds (C-H) are scattered throughout the glucose molecule. These bonds also store a significant amount of energy. They are slightly weaker than C-C bonds, but their abundance makes their contribution to the overall energy content of glucose considerable. The oxidation of these bonds during cellular respiration is a key step in energy extraction.
3. Carbon-Oxygen Bonds:
The presence of carbon-oxygen bonds (C-O) in the glucose molecule, specifically in the hydroxyl (-OH) groups, adds complexity to its energy storage. While not as energy-rich as C-C or C-H bonds, the C-O bonds contribute to the molecule's overall stability and participate in important biochemical reactions. The rearrangement of these bonds during cellular respiration facilitates energy release.
4. High-Energy Phosphate Bonds:
While glucose itself doesn't directly contain high-energy phosphate bonds like ATP (adenosine triphosphate), its metabolism leads to the formation of these bonds, which are the actual currency of cellular energy. The energy released from the breakdown of glucose is used to create high-energy phosphate bonds in ATP, providing the immediate energy needed for cellular work. This process is a crucial link between the energy stored in glucose and its utilization by the cell.
Cellular Respiration: Unlocking the Energy
The energy stored within the glucose molecule isn't directly accessible to the cell. It requires a series of controlled biochemical reactions, collectively known as cellular respiration, to release it. This process takes place in several stages:
1. Glycolysis:
Glycolysis occurs in the cytoplasm and is an anaerobic process (doesn't require oxygen). It involves the breakdown of glucose into two molecules of pyruvate. This process involves breaking some C-C and C-H bonds, releasing a small amount of energy that is used to generate a net gain of two ATP molecules. This is only a fraction of the total energy stored in glucose.
2. Pyruvate Oxidation:
Pyruvate, the product of glycolysis, is transported into the mitochondria, the powerhouse of the cell. Here, it undergoes oxidation, converting into acetyl-CoA. This step releases more energy and produces NADH, an electron carrier crucial for the next stage.
3. Krebs Cycle (Citric Acid Cycle):
The acetyl-CoA enters the Krebs cycle, a series of reactions that completely oxidize the carbon atoms in the original glucose molecule to carbon dioxide. This oxidation releases more energy, generating ATP, NADH, and FADH2 (another electron carrier). The Krebs cycle efficiently extracts energy from the remaining bonds within the glucose molecule's carbon backbone.
4. Oxidative Phosphorylation (Electron Transport Chain & Chemiosmosis):
This is the final and most energy-yielding stage. The NADH and FADH2 generated in the previous steps deliver their electrons to the electron transport chain (ETC), a series of protein complexes embedded in the inner mitochondrial membrane. As electrons move down the ETC, energy is released and used to pump protons (H⁺) across the membrane, creating a proton gradient. This gradient drives ATP synthase, an enzyme that synthesizes a large amount of ATP from ADP and inorganic phosphate. This process, called chemiosmosis, is remarkably efficient in converting the energy from electron transfer into the high-energy phosphate bonds of ATP. The oxygen we breathe is the final electron acceptor in the ETC, forming water.
Beyond Glucose: Other Energy Storage Molecules
While glucose is a primary energy source, cells utilize other molecules for energy storage, particularly for longer-term needs:
1. Glycogen:
Animals store glucose in the form of glycogen, a highly branched polysaccharide. Glycogen is primarily stored in the liver and muscles, serving as a readily available source of glucose when blood sugar levels drop. The energy is essentially stored in the numerous glycosidic bonds linking glucose units within the glycogen molecule. These bonds are readily hydrolyzed to release glucose when needed.
2. Starch:
Plants store glucose as starch, another polysaccharide, primarily in the form of amylose and amylopectin. Starch serves as a long-term energy reserve for plants. Similar to glycogen, the energy is stored within the glycosidic bonds linking the glucose units. The breakdown of starch, like glycogen, releases glucose for cellular respiration.
3. Fats (Triglycerides):
Fats, or triglycerides, are the most efficient form of long-term energy storage in both animals and plants. They store much more energy per gram than carbohydrates or proteins. The energy is stored in the ester bonds connecting fatty acids to glycerol, as well as within the long hydrocarbon chains of the fatty acids themselves. The oxidation of these fatty acids releases a vast amount of energy during cellular respiration.
Conclusion: A Complex Dance of Energy Transfer
The energy stored in glucose isn't located in a single, easily identifiable spot. It's distributed throughout the molecule's intricate structure, primarily within its carbon-carbon and carbon-hydrogen bonds. The cellular machinery meticulously extracts this energy through the tightly regulated processes of cellular respiration. This intricate dance of biochemical reactions transforms the potential energy locked within glucose into the readily usable energy of ATP, fueling all cellular activities and powering life itself. Understanding the precise location and mechanisms of energy storage in glucose is fundamental to comprehending the intricate biochemical processes that sustain life. Further research into these processes continues to unlock more detailed insights into this fundamental aspect of biological energy.
Latest Posts
Latest Posts
-
Is Density And Specific Gravity The Same
Mar 30, 2025
-
Which Complex Carbohydrate Contains Only A 1 4 Glycosidic Linkages
Mar 30, 2025
-
Understanding Media And Culture An Introduction To Mass Communication
Mar 30, 2025
-
The Substance That Is Being Dissolved By A Solvent
Mar 30, 2025
-
What Percentage Of Alcohol Is Absorbed Through The Small Intestine
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Where Is This Energy Stored In Glucose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
