Calculating The Ph Of A Strong Acid Solution
Muz Play
Mar 25, 2025 · 5 min read
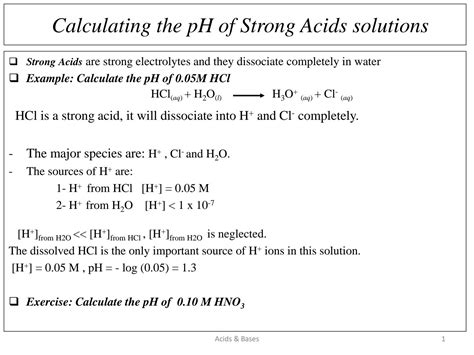
Table of Contents
Calculating the pH of a Strong Acid Solution: A Comprehensive Guide
Determining the pH of a solution is a fundamental concept in chemistry with widespread applications in various fields, from environmental monitoring to industrial processes. Understanding how to calculate the pH, especially for strong acids, is crucial for anyone working with chemical solutions. This comprehensive guide will walk you through the process, covering the underlying principles, step-by-step calculations, and common pitfalls to avoid.
Understanding pH and Strong Acids
Before diving into calculations, let's refresh our understanding of key concepts.
What is pH?
pH is a measure of the hydrogen ion concentration ([H⁺]) in a solution, indicating its acidity or alkalinity. The pH scale ranges from 0 to 14, where:
- pH < 7: Indicates an acidic solution (higher [H⁺]).
- pH = 7: Indicates a neutral solution.
- pH > 7: Indicates an alkaline (or basic) solution (lower [H⁺]).
The pH is calculated using the following formula:
pH = -log₁₀[H⁺]
This means the pH is the negative logarithm (base 10) of the hydrogen ion concentration. A lower pH value signifies a higher concentration of H⁺ ions.
Strong Acids: Complete Dissociation
A strong acid is an acid that completely dissociates in water. This means that when a strong acid is dissolved in water, all of its molecules donate a proton (H⁺) to water molecules, forming hydronium ions (H₃O⁺). For practical purposes, we can consider [H⁺] and [H₃O⁺] to be equivalent. Examples of common strong acids include:
- Hydrochloric acid (HCl)
- Sulfuric acid (H₂SO₄)
- Nitric acid (HNO₃)
- Perchloric acid (HClO₄)
- Hydrobromic acid (HBr)
- Hydroiodic acid (HI)
Calculating the pH of a Strong Acid Solution: A Step-by-Step Approach
The calculation of pH for a strong acid solution is relatively straightforward due to the complete dissociation. Let's consider a few examples to illustrate the process.
Example 1: Monoprotic Strong Acid (HCl)
Let's calculate the pH of a 0.1 M solution of hydrochloric acid (HCl). HCl is a monoprotic strong acid, meaning it donates one proton per molecule.
Step 1: Write the dissociation equation.
HCl(aq) → H⁺(aq) + Cl⁻(aq)
Step 2: Determine the hydrogen ion concentration.
Since HCl completely dissociates, the concentration of H⁺ ions is equal to the initial concentration of HCl.
[H⁺] = 0.1 M
Step 3: Calculate the pH.
pH = -log₁₀[H⁺] = -log₁₀(0.1) = 1
Therefore, the pH of a 0.1 M HCl solution is 1.
Example 2: Diprotic Strong Acid (H₂SO₄)
Sulfuric acid (H₂SO₄) is a diprotic strong acid, meaning it donates two protons per molecule. However, the second dissociation is not always complete and must be taken into consideration for higher concentrations. For dilute solutions, it's generally acceptable to consider only the first dissociation as complete.
Let's calculate the pH of a 0.05 M solution of H₂SO₄, assuming only the first dissociation is complete.
Step 1: Write the first dissociation equation.
H₂SO₄(aq) → H⁺(aq) + HSO₄⁻(aq)
Step 2: Determine the hydrogen ion concentration.
[H⁺] = 0.05 M (assuming only the first proton dissociates completely)
Step 3: Calculate the pH.
pH = -log₁₀[H⁺] = -log₁₀(0.05) ≈ 1.3
Therefore, the pH of a 0.05 M H₂SO₄ solution (approximating only the first dissociation) is approximately 1.3. For more accurate calculations at higher concentrations, the second dissociation constant (Ka2) would need to be incorporated.
Example 3: Strong Acid Dilution
Understanding how dilution affects pH is essential. Let's consider diluting 10 mL of 1 M HCl to a final volume of 100 mL with water.
Step 1: Calculate the moles of HCl.
Moles of HCl = (Concentration) x (Volume) = 1 M x 0.01 L = 0.01 moles
Step 2: Calculate the new concentration after dilution.
New concentration = (Moles of HCl) / (Final Volume) = 0.01 moles / 0.1 L = 0.1 M
Step 3: Calculate the pH.
[H⁺] = 0.1 M
pH = -log₁₀(0.1) = 1
The pH remains 1; only the volume changes, and concentration remains the same. This illustrates the importance of recognizing the impact of dilution on concentration.
Advanced Considerations: Polyprotic Acids and Activity Coefficients
The examples above focus on simpler scenarios. Let's explore more complex situations:
Polyprotic Strong Acids: Beyond the First Dissociation
For polyprotic strong acids like sulfuric acid (H₂SO₄), the second dissociation is not as complete as the first. While the first proton dissociates completely, the second dissociation needs to be handled with the appropriate equilibrium constant (Ka2). This requires solving a quadratic equation, making the calculation more involved.
Activity Coefficients: Accounting for Ionic Strength
In concentrated solutions, the interaction between ions affects their effective concentration. This is taken into account using activity coefficients. The activity (a) of an ion is related to its concentration (c) by:
a = γc
where γ is the activity coefficient, a value less than 1 that accounts for interionic interactions. Calculating activity coefficients requires specialized methods and consideration of the ionic strength of the solution. This further complicates pH calculations for concentrated strong acid solutions.
Common Mistakes and How to Avoid Them
Several common mistakes can lead to inaccurate pH calculations:
- Ignoring the complete dissociation: For strong acids, remember that the assumption of complete dissociation is crucial for the calculation.
- Incorrect use of significant figures: Pay close attention to significant figures throughout your calculations to avoid errors in the final pH value.
- Failing to consider the stoichiometry: For polyprotic acids, correctly handling the stoichiometry of each dissociation step is essential.
- Neglecting activity coefficients: In concentrated solutions, ignoring activity coefficients can lead to significant errors.
Conclusion
Calculating the pH of a strong acid solution is a fundamental skill in chemistry. While the basic calculation is straightforward for dilute solutions of monoprotic strong acids, more complex scenarios involving polyprotic acids and concentrated solutions require more advanced techniques and consideration of activity coefficients. By understanding the underlying principles and carefully following the steps outlined in this guide, you can confidently tackle a wide range of pH calculations. Remember to always consider the specific context of your problem and the potential need for more sophisticated methods to ensure accuracy.
Latest Posts
Latest Posts
-
Leave As Is To A Writer
Mar 26, 2025
-
Cell Membrane And Transport Coloring Answer Key
Mar 26, 2025
-
What Are Elements In Group 17 Called
Mar 26, 2025
-
Factoring The Greatest Common Monomial Factor
Mar 26, 2025
-
Vascular Anatomy Of The Lower Extremity
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Calculating The Ph Of A Strong Acid Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
