What Are Elements In Group 17 Called
Muz Play
Mar 26, 2025 · 7 min read
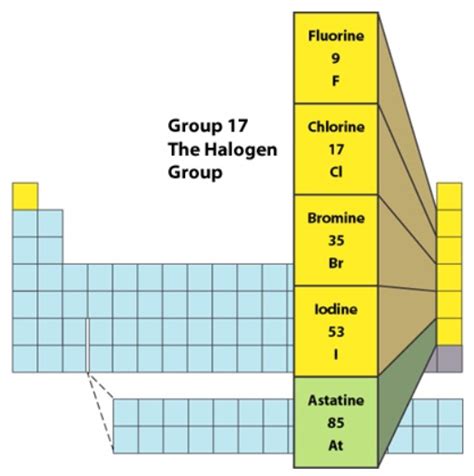
Table of Contents
What are Elements in Group 17 Called? Exploring the Halogens
The elements in Group 17 of the periodic table are collectively known as halogens. This name, derived from the Greek words "hals" (salt) and "genes" (forming), perfectly encapsulates their defining characteristic: their tendency to readily form salts with metals. Understanding the halogens goes beyond simply knowing their name; it involves delving into their unique properties, reactivity, and diverse applications. This comprehensive guide will explore these fascinating elements in detail.
The Halogen Family: A Closer Look at Fluorine, Chlorine, Bromine, Iodine, and Astatine
The halogen family consists of five elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). While all share fundamental similarities, their properties vary significantly as you move down the group. This variation is a direct consequence of their increasing atomic radius and the decreasing electronegativity.
1. Fluorine (F): The Most Reactive Halogen
Fluorine, the lightest and smallest halogen, holds the distinction of being the most reactive non-metal. Its incredibly high electronegativity – the ability to attract electrons in a chemical bond – makes it exceptionally aggressive in its reactions. Pure fluorine is a pale yellow, highly corrosive gas. It reacts violently with almost all other elements, even noble gases under specific conditions. Its reactivity stems from its small size and its strong pull on electrons.
Key Characteristics of Fluorine:
- Extremely reactive: Reacts readily with most substances, including water.
- Pale yellow gas: Exists as a diatomic molecule (F₂).
- High electronegativity: The highest of all elements.
- Strong oxidizing agent: Easily gains electrons.
- Applications: Production of fluorocarbons (e.g., Teflon), in the enrichment of uranium, and in toothpaste as a source of fluoride ions for dental health.
2. Chlorine (Cl): A Versatile and Abundant Halogen
Chlorine, a greenish-yellow gas, is significantly less reactive than fluorine but still highly reactive. It's readily available and is widely used in various industrial processes. Chlorine plays a crucial role in water purification, acting as a powerful disinfectant. However, its high reactivity makes it necessary to handle it cautiously due to its potentially harmful nature.
Key Characteristics of Chlorine:
- Greenish-yellow gas: Exists as a diatomic molecule (Cl₂).
- Strong oxidizing agent: But less powerful than fluorine.
- Versatile reactivity: Reacts with many elements and compounds.
- Applications: Water purification, bleaching agent, production of PVC (polyvinyl chloride), and other chemicals.
- Environmental concerns: Chlorine compounds can contribute to ozone depletion and acid rain.
3. Bromine (Br): The Only Liquid Non-metal
Bromine is unique among the halogens as it's the only one that exists as a liquid at room temperature. It's a dense, reddish-brown liquid with a pungent and irritating vapor. Bromine is less reactive than chlorine and fluorine but still exhibits significant reactivity. Its applications are diverse, including use in agricultural chemicals and flame retardants. However, its toxicity mandates careful handling.
Key Characteristics of Bromine:
- Reddish-brown liquid: Exists as a diatomic molecule (Br₂).
- Moderately reactive: Less reactive than chlorine and fluorine.
- Volatile and pungent: Produces irritating vapors.
- Applications: Production of flame retardants, agricultural chemicals, and dyes.
- Toxicity: Requires careful handling due to its corrosive and toxic nature.
4. Iodine (I): A Shiny, Dark Grey Solid
Iodine is a shiny, dark grey solid that sublimes readily—meaning it transitions directly from a solid to a gas—forming a violet vapor. It's significantly less reactive than the lighter halogens. Iodine plays a vital role in human physiology, as it's essential for the production of thyroid hormones.
Key Characteristics of Iodine:
- Dark grey solid: Sublimes to form a violet vapor.
- Less reactive: Compared to lighter halogens.
- Essential nutrient: Crucial for thyroid hormone production.
- Applications: Antiseptic, dietary supplement, and in photography.
5. Astatine (At): A Radioactive and Scarce Element
Astatine is a radioactive element, making it significantly different from the other halogens. It's extremely rare and has a short half-life, preventing extensive study of its properties. Its chemical properties are expected to be similar to iodine, but its radioactivity makes it extremely hazardous.
Key Characteristics of Astatine:
- Radioactive: Highly unstable with a short half-life.
- Extremely rare: Trace amounts found in uranium ores.
- Predicted properties: Similar to iodine, but its radioactivity dominates its characteristics.
- Limited applications: Primarily used in research due to its rarity and radioactivity.
The Periodic Trends in Halogens: A Systematic Overview
The properties of halogens exhibit clear trends as you move down the group in the periodic table. These trends are directly related to the increasing atomic size and decreasing electronegativity.
-
Atomic Radius: The atomic radius increases down the group, as electrons are added to higher energy levels further from the nucleus. This increased distance weakens the attraction between the nucleus and the outermost electrons.
-
Electronegativity: Electronegativity decreases down the group. The larger atomic radius means the nucleus's attraction to additional electrons is weaker, resulting in reduced electronegativity.
-
Ionization Energy: Ionization energy (the energy required to remove an electron) decreases down the group, reflecting the weaker attraction between the nucleus and the outermost electrons.
-
Melting and Boiling Points: The melting and boiling points increase down the group. This is due to increased intermolecular forces (van der Waals forces) between the larger atoms.
Reactions of Halogens: A Chemical Perspective
Halogens are highly reactive due to their high electronegativity and their tendency to gain one electron to achieve a stable electron configuration (octet rule). Their reactions can be broadly classified into:
-
Reactions with Metals: Halogens readily react with metals to form ionic compounds, also known as halides. For instance, the reaction of sodium (Na) with chlorine (Cl₂) produces sodium chloride (NaCl), common table salt.
-
Reactions with Non-Metals: Halogens also react with non-metals, but these reactions often involve covalent bonding. For example, chlorine reacts with hydrogen (H₂) to form hydrogen chloride (HCl), a strong acid.
-
Displacement Reactions: A more reactive halogen can displace a less reactive halogen from its compound. For example, chlorine can displace bromine from a bromide solution: Cl₂ + 2NaBr → 2NaCl + Br₂
-
Reactions with Water: Fluorine reacts violently with water, while chlorine reacts to form a mixture of hydrochloric acid (HCl) and hypochlorous acid (HOCl). Bromine and iodine react slowly with water.
Applications of Halogens: From Everyday Use to Advanced Technology
The halogens find a vast range of applications in diverse fields, reflecting their unique chemical properties:
-
Industrial Applications: Chlorine is used extensively in water purification, bleaching, and the production of various chemicals, including PVC. Bromine is used in flame retardants and agricultural chemicals.
-
Medical Applications: Iodine is used as an antiseptic and in some medications. Fluoride ions, derived from fluorine, are added to toothpaste and drinking water to prevent tooth decay.
-
Household Applications: Many household cleaning products contain chlorine or hypochlorite compounds.
-
Advanced Technologies: Fluorine is used in the production of fluorocarbons, which have applications in refrigeration, air conditioning, and non-stick cookware (Teflon). Halogens are also used in various specialized applications like laser technology and photography.
Environmental Impact of Halogens: A Balancing Act
While halogens have numerous beneficial applications, their use also raises environmental concerns:
-
Ozone Depletion: Certain chlorofluorocarbons (CFCs) and halons, which contain chlorine and bromine, have been linked to ozone depletion in the stratosphere. International agreements, such as the Montreal Protocol, have phased out the production and use of many of these ozone-depleting substances.
-
Acid Rain: Some halogen compounds can contribute to acid rain, which can have detrimental effects on ecosystems and human health.
-
Toxicity: Many halogen compounds are toxic, and proper handling and disposal are essential to minimize environmental risks.
Conclusion: The Significance of Halogens in Our World
The elements in Group 17, the halogens, represent a fascinating group of non-metals with diverse properties and applications. From their role in essential biological processes to their use in various industrial and technological applications, halogens are integral to our world. Understanding their unique reactivity, periodic trends, and environmental impact is crucial for responsible utilization and minimizing potential risks. Further research continues to unravel the complexities of these remarkable elements and their ever-evolving applications.
Latest Posts
Latest Posts
-
How Do You Calculate The Average Acceleration
Mar 26, 2025
-
What Subatomic Particles Make Up An Atom
Mar 26, 2025
-
What Is A Role Conflict In Sociology
Mar 26, 2025
-
What Is The Trend Of Atomic Radii
Mar 26, 2025
-
How To Draw Electron Dot Diagrams
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Are Elements In Group 17 Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
