What Is The Trend Of Atomic Radii
Muz Play
Mar 26, 2025 · 5 min read
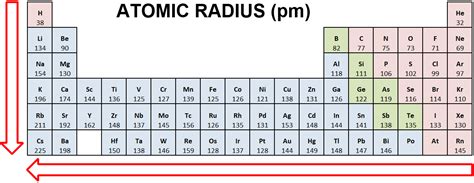
Table of Contents
The Trend of Atomic Radii: A Comprehensive Exploration
Atomic radius, a fundamental property in chemistry, dictates many aspects of an element's behavior and reactivity. Understanding its trends across the periodic table is crucial for predicting chemical properties and interpreting experimental observations. This article delves deep into the trends of atomic radii, exploring the underlying reasons behind these patterns and their implications. We'll cover the factors influencing atomic size, variations within periods and groups, and the exceptions to the general rules.
What is Atomic Radius?
Atomic radius isn't a precisely defined value, as the electron cloud surrounding the nucleus doesn't have a sharp boundary. Instead, we utilize several methods to approximate atomic size. These include:
- Metallic Radius: Half the distance between the nuclei of two adjacent atoms in a metallic crystal. This is applicable to metals only.
- Covalent Radius: Half the distance between the nuclei of two identical atoms bonded covalently. This applies to nonmetals and some metalloids.
- Van der Waals Radius: Half the distance between the nuclei of two identical atoms that are not chemically bonded but are in close proximity. This applies to non-bonded atoms.
It's important to note that the specific method used to determine atomic radius can influence the numerical value, but the overall trends remain consistent. Throughout this article, we will discuss atomic radius in a general sense, encompassing these different definitions.
Trends in Atomic Radius Across the Periodic Table
The periodic table's organization reflects the systematic trends in atomic properties, and atomic radius is no exception. These trends are primarily driven by two key factors:
-
Effective Nuclear Charge: This is the net positive charge experienced by an electron in a multi-electron atom. It's the difference between the number of protons in the nucleus (nuclear charge) and the number of shielding electrons (electrons in inner shells). A higher effective nuclear charge pulls electrons closer to the nucleus, resulting in a smaller atomic radius.
-
Principal Quantum Number (n): This quantum number determines the electron shell's energy level. As n increases, the electron is further from the nucleus, leading to a larger atomic radius. Higher energy levels correspond to larger orbitals.
Trend Across a Period (Left to Right)
As we move across a period from left to right, the atomic number increases, meaning more protons are added to the nucleus. Electrons are added to the same principal energy level (same n value). While the number of shielding electrons increases slightly, the increase in nuclear charge is more significant. This leads to a stronger pull on the electrons, resulting in a decrease in atomic radius across a period.
Trend Down a Group (Top to Bottom)
Moving down a group, we add electrons to successively higher principal energy levels (n increases). The increased distance of the outermost electrons from the nucleus overrides the effect of the increased nuclear charge. Therefore, the atomic radius increases down a group. The addition of new electron shells significantly outweighs the increase in effective nuclear charge.
Exceptions to the General Trends
While the general trends are well-established, there are some notable exceptions. These deviations often arise from electron-electron repulsions or subtle variations in effective nuclear charge. Examples include:
-
Transition Metals: The slight increase in atomic radius across some transition metal series can be attributed to the poor shielding effect of d-electrons. The gradual addition of electrons to the d subshell doesn't always lead to a proportional increase in shielding, thus partially counteracting the increase in nuclear charge.
-
Lanthanide Contraction: The unexpectedly small atomic radii of the lanthanides (elements 57-71) are due to the poor shielding effect of the 4f electrons. The increased nuclear charge isn't effectively shielded, resulting in a stronger pull on the outer electrons and smaller atomic radii. This contraction has a significant knock-on effect on the subsequent elements in the periodic table.
-
Actinide Contraction: Similar to the lanthanides, the actinides (elements 89-103) also exhibit a contraction in atomic radii due to the poor shielding of the 5f electrons.
These exceptions demonstrate the complexities of atomic structure and the interplay of different forces influencing atomic size.
The Significance of Atomic Radius Trends
Understanding the trends in atomic radii is crucial for various aspects of chemistry and related fields:
-
Chemical Reactivity: Atomic radius influences the distance between atoms in molecules and thus affects bond lengths and bond energies. Smaller atoms generally form stronger bonds.
-
Ionization Energy: The energy required to remove an electron from an atom is inversely related to atomic radius. Smaller atoms with higher effective nuclear charge have higher ionization energies.
-
Electron Affinity: The ability of an atom to accept an electron is also influenced by atomic radius. Smaller atoms can attract electrons more strongly.
-
Metallic Character: Metallic character tends to increase with atomic radius as larger atoms have less tightly bound outer electrons.
-
Crystal Structure: Atomic radii dictate the packing arrangements of atoms in crystals and thus influence the overall properties of solid materials.
-
Predicting Chemical Properties: By understanding the trends in atomic radii, chemists can make predictions about the reactivity and chemical behavior of different elements.
Advanced Considerations: Relativistic Effects
For heavier elements, particularly those with high atomic numbers, relativistic effects become increasingly significant. Relativistic effects arise from the high speeds of inner-shell electrons, causing them to have a greater mass and move closer to the nucleus. This leads to a contraction of the inner electron shells, which in turn can affect the outer electron shells and ultimately the atomic radius. This effect is especially pronounced for elements like gold and mercury, where the relativistic contraction leads to unexpected properties such as the gold's color and mercury's liquid state at room temperature.
Conclusion: A Dynamic Property
Atomic radius is a dynamic property that reflects the complex interplay of several fundamental forces within an atom. While general trends exist, exceptions highlight the nuances of electron behavior and the limitations of simplified models. Understanding these trends and exceptions is fundamental to appreciating the periodic table's organization and to predicting and interpreting the chemical and physical properties of the elements. Further research continues to refine our understanding of atomic radii and its influence on chemical phenomena, incorporating increasingly sophisticated theoretical and experimental techniques. The study of atomic radii remains an essential component of modern chemistry, driving advancements in materials science, theoretical chemistry, and our overall understanding of the elements and their interactions.
Latest Posts
Latest Posts
-
Definition Of Order Of A Reaction
Mar 29, 2025
-
Why Is Blood Clotting A Positive Feedback
Mar 29, 2025
-
What Elements Are Most To Become Anions
Mar 29, 2025
-
Which Place On An Enzyme Binds A Substrate
Mar 29, 2025
-
The Number Of Electrons Moving Is Known As
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Trend Of Atomic Radii . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
